Figure 2.
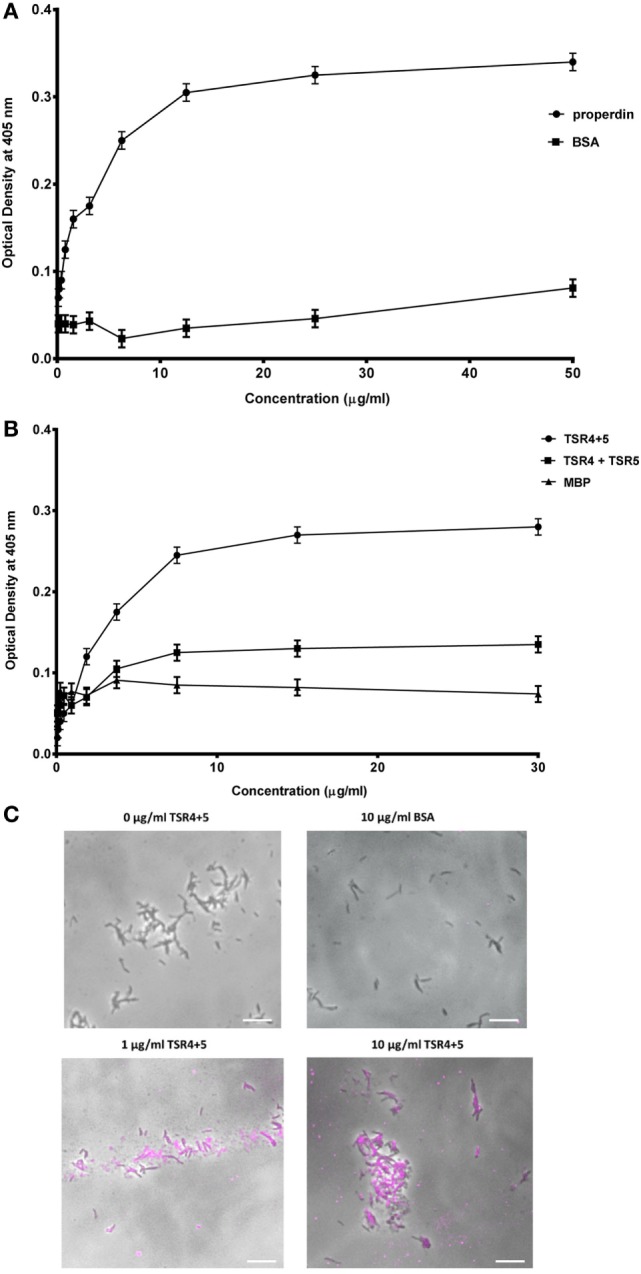
Human properdin binds mycobacteria via thrombospondin repeats (TSR) 4+5. (A) Properdin binding to mycobacteria; BSA was used as a negative control protein. (B) Comparison between TSR4+5 and individual TSR4 and TSR5 binding to mycobacteria; maltose-binding protein (MBP) as negative control. Assays were conducted in 10 mM HEPES, 140 mM NaCl, 0.5 mM CaCl2 + 0.5 mM MgCl2, 100 µg/ml hen ovalbumin, and pH 7.5. Serial dilutions of properdin were incubated in mycobacteria coated wells followed by incubation with mouse anti-properdin monoclonal antibody and mouse anti-BSA monoclonal antibody, respectively; serial dilutions of TSR4+5, TSR4 or TSR5 were incubated in another set of mycobacteria coated wells followed by incubation with mouse anti-MBP monoclonal antibody. Anti-mouse IgG conjugated with alkaline phosphatase and substrate p-nitrophenol phosphate were incubated in both sets of wells, and the color was measured at 405 nm using a plate reader. Assay was conducted in quadruplicate. Error bars represent SD. (C) Differential direct binding of 0, 1, and 10 µg/ml of TSR4+5 to Mycobacterium bovis BCG. 10 µg/ml of BSA was used as a negative control. Cells were incubated for 2 h with either TSR4+5 or BSA. Cells were washed, fixed, and stained with mouse anti-MBP monoclonal antibody followed by goat anti-mouse 1gG-conjugated with AlexaFluor488. Images are shown as single sections taken using a Leica DM4000 microscope; bar scale 10 µm.
