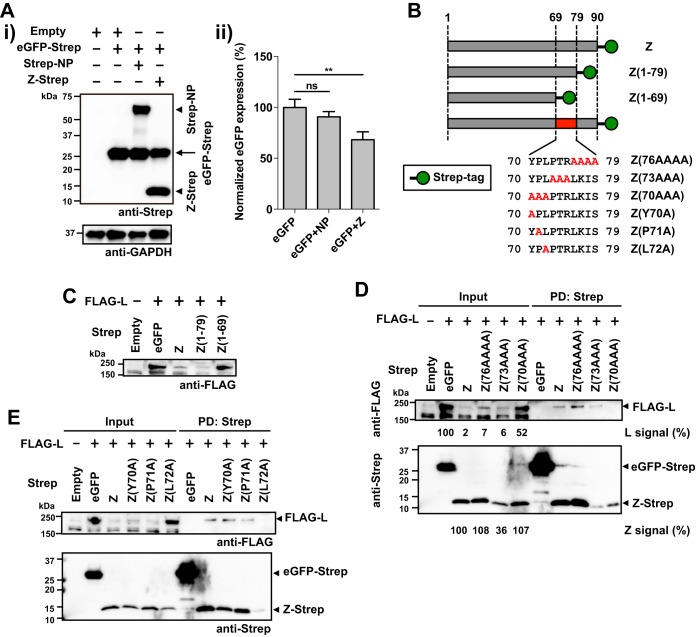
FIG 2.
The leucine (L) residue at position 72 of LCMV Z is critical for Z-L interaction. (A) Reduction of eGFP expression by coexpression with the Z protein. 293T cells were transfected with 0.5 μg of pC-eGPF-Strep together with 0.5 μg of pC-Empty (Empty), pC-Strep-NP (Strep-NP), or pC-Z-Strep (Z-Strep). At 48 h posttransfection, cell lysates were prepared and protein expression was examined by Western blotting. Representative Western blot data from three independent experiments are shown (i). Data represent means ± SD of results from three independent experiments (ii). Mean signal intensity of eGPF-Strep expressed alone was set to 100%. ** P value of < 0.01. ns, not significant. (B) Schematic diagram of amino acid composition of the C termini of the WT and mutant Z proteins. (C to E) Mapping of amino acid residues required for Z-L interaction. 293T cells were cotransfected with FLAG-L and Strep-tagged WT or the indicated mutant Z, and at 48 hm posttransfection cell lysates were prepared and protein expression in whole-cell lysates and Strep tag-mediated PD was examined as described for Fig. 1. Numbers at the bottoms of the anti-FLAG and anti-Strep Western blots correspond to relative signal intensity of L [L signal (%)] or Z protein [Z signal (%)], respectively, in whole-cell lysate (Input) (D). Signal intensity of FLAG-tagged L protein coexpressed with eGFP-Strep or that of Strep-tagged WT Z protein was set to 100%.