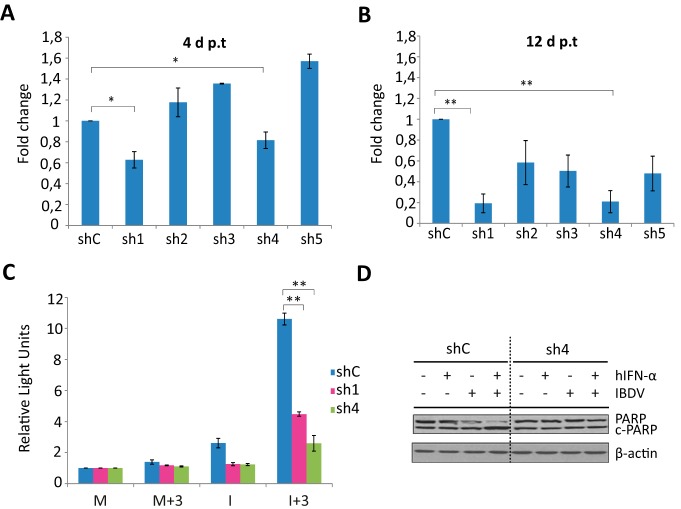
FIG 5.
Effect of TNF-α knockdown on triggering of apoptosis by IFN in IBDV-infected HeLa cells. (A and B) TNF-α silencing by shRNAs. HeLa cells were transduced with lentiviral vectors expressing an irrelevant sequence (shC) or shRNAs targeting TNF-α mRNA (sh1 to sh5). At 4 days p.t. (A) and 12 days p.t. (B), cells were transfected with poly(I·C) for 16 h to induce TNF-α gene expression, and the levels of TNF-α mRNA were determined by SYBR green-based RT-qPCR. The results of TNF-α gene expression levels, normalized against the HPRT mRNA level, are presented as fold changes relative to the level in HeLa shC cells transfected with poly(I·C). Bars indicate means ± standard deviations based on data from triplicate samples. * indicates a P value of <0.05, as determined by unpaired Student's t test. (C and D) Effect of TNF-α silencing on apoptosis. HeLa cells transduced with lentiviral vectors expressing shC, sh1, and sh4 were mock infected or infected with IBDV at an MOI of 2 at 12 days p.t. and treated (+) or not (−) with 1,000 IU/ml of hIFN-α at 3 h p.i. Cells were harvested at 24 h p.i. (C) Apoptosis was measured by using the Caspase-Glo 3/7 assay kit. Each determination was carried out in duplicate. The presented data correspond to the means ± the standard deviations of results from two independent experiments. Caspase values for infected cell samples were normalized to those for mock-infected cells. Bars indicate means ± standard deviations based on data from duplicate samples. * and ** indicate P values of <0.05 and <0.01, respectively, as determined by unpaired Student's t test. (D) PARP cleavage analyzed by Western blotting. Total cell extracts were subjected to SDS-PAGE, transferred to nitrocellulose, and immunoblotted with serum anti-PARP. The PARP cleavage product is denoted c-PARP. The Western blot corresponding to β-actin was used as a protein loading control.