Figure 8.
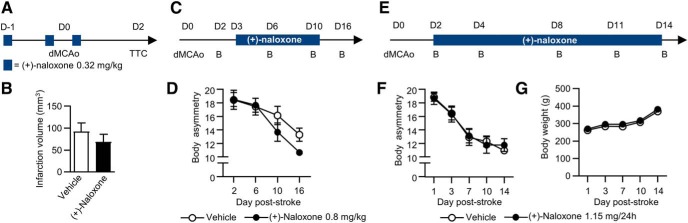
Effect of different pre- and post-stroke treatment with (+)-naloxone on infarct volume and functional recovery. A, Experimental timeline. Intranasal (+)-naloxone or vehicle was administered three times: 12 and 1 h before dMCAo and immediately after reperfusion. Infarction volume was determined by TTC staining 2 d after stroke. B, Average infarction volume (mm3) on day 2 post-stroke in vehicle (n = 7) and (+)-naloxone (0.32 mg/kg; n = 8) pretreated rats. Prestroke intranasal administration of (+)-naloxone was not neuroprotective in 60-min dMCAo. C, Experimental timeline. (+)-Naloxone was delivered intranasally twice daily for 7 d post-stroke starting from post-stroke day 3. D, The effect of (+)-naloxone (0.8 mg/kg; n = 6) and vehicle (n = 7) treatment from post-stroke day 3 to post-stroke day 10 on body asymmetry test. E, Experimental timeline. (+)-Naloxone was delivered into the ventricle via mini-osmotic pumps for 12 d post-stroke starting from post-stroke day 2. F, G, The effects of 12-d continuous delivery of (+)-naloxone (1.15 mg/24 h; n = 7) and vehicle (n = 8) on body asymmetry test (F) and body weight (G). In A, C, and E: D, indicated post-stroke day; B, behavioral assay. The data represent mean ± SEM.