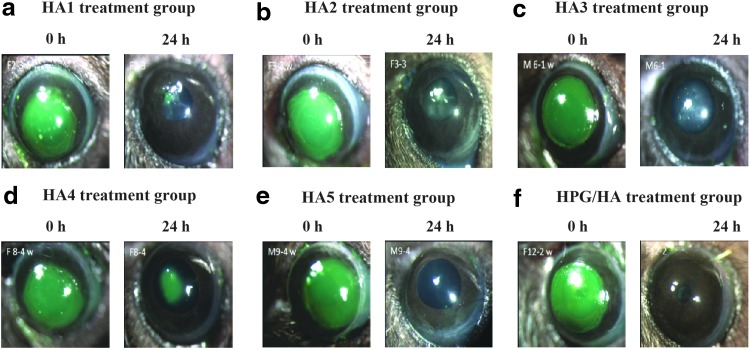
FIG. 2.
Representative images showing corneal epithelium before and 24 h post debridement in each treatment group (a-f) (green staining on images indicate wound area). HA, hyaluronic acid; HA1, Optive Fusion™, Allergan Limited, United Kingdom; HA2, Vismed®, TRB Chemedica International S.A., Switzerland; c) HA3, Thealoz Duo®, Thea Pharmaceuticals Limited, United Kingdom; HA4, Hyabak®, Thea Pharmaceuticals Limited, United Kingdom; HA5, Hylo-Comod®, URSAPHARM Arzneimittel GmbH, Germany; and HPG/HA, Systane® Hydration, Alcon Research Ltd., Fort Worth Texas, USA; HPG/HA, hydroxypropyl guar/hyaluronic acid-containing dual-polymer lubricant formulation.