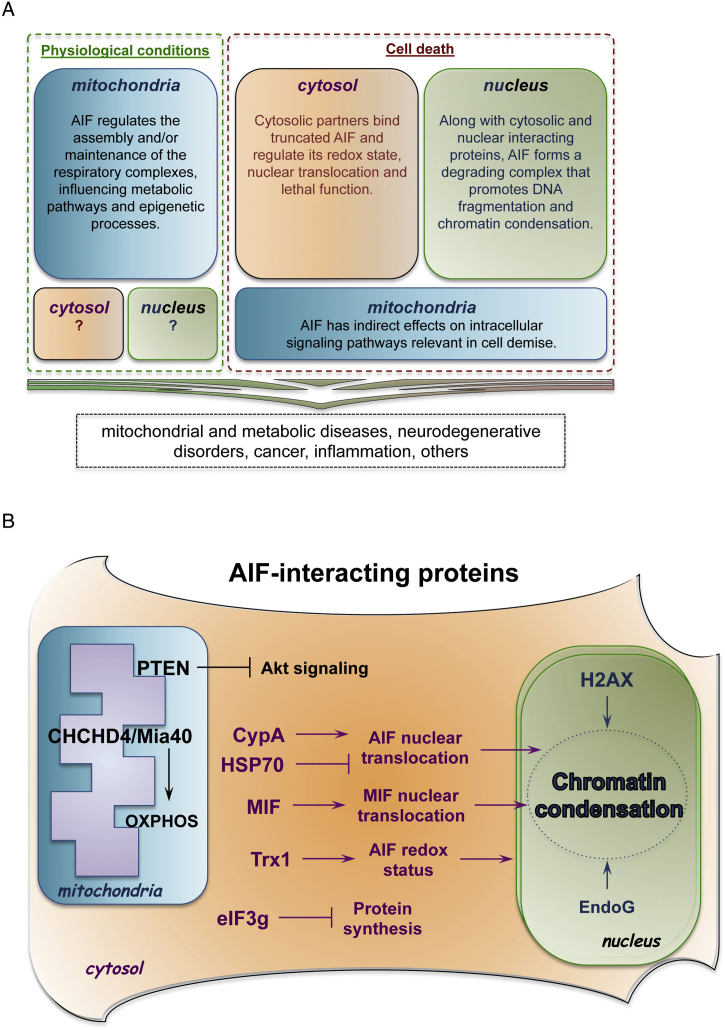
Fig. 1.
Pathophysiological functions of AIF. (A) Under physiological settings, AIF has a vital role in mitochondrial bioenergetics, since it supports the normal oxidative phosphorylation of the cell. Consequently, mitochondrial AIF has an impact on multiple catabolic and anabolic pathways, as well as on epigenetic processes that depend on mitochondrial metabolites. It remains unclear whether soluble AIF molecules are present in other subcellular compartments under physiological conditions. Upon detrimental signals, mitochondrial AIF indirectly modulates intracellular signaling pathways (e.g., PTEN/Akt), while cytosolic AIF binds molecular partners that determine its nuclear translocation. In the nucleus, AIF forms a degrading complex responsible for chromatinolysis and cell death. These molecular processes have been associated to an array of pathological conditions, including inherited diseases. (B) Schematic summaries of AIF-interacting proteins. Mitochondrial intermembrane space: AIF binding to CHCHD4/Mia40 contributes to the oxidative folding of electron transport chain subunits. Moreover, AIF physically interacts with mitochondria-localized PTEN, inhibits PTEN oxidation and indirectly influences Akt signaling pathway. Cytosol: upon release from the mitochondria, AIF interacts with several cytosolic proteins. The binding kinetics between HSP70 and CypA determines AIF nuclear translocation rate. In the cytosol and in the nucleus, TRX1 interaction prevents AIF oxidation in cells exposed to stress and undergoing apoptosis. Another recently identified AIF-binding partner is MIF. In a functional complex with AIF, the endonuclease MIF translocates to the nucleus and mediates chromatinolysis. During apoptosis, AIF interacts with the eukaryotic translational initiation factor EIF3g and, consequently, inhibits protein synthesis. Nucleus: along with CypA, AIF interacts with histone H2AX and forms a degrading complex that regulates DNA cleavage. At least in invertebrates, AIF regulates EndoG activity and promotes chromatin condensation.