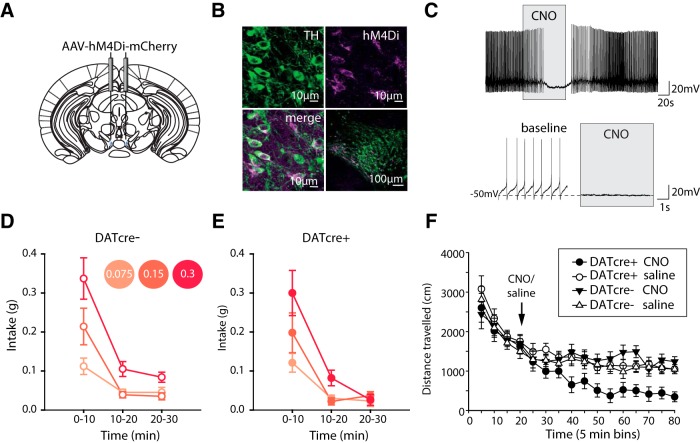
Figure 2.
Chemogenetic inhibition of VTA dopamine neurons does not affect intake of high-concentration salt jellies during salt appetite. A, DATcre+ mice (n = 9) and DATcre- mice (n = 11) were injected in the VTA with cre-dependent AAV carrying the Gi-coupled human M4 muscarinic DREADD coding sequence conjugated to the fluorescent protein mCherry (AAV-hM4Di-mCherry). B, Colocalization of mCherry and TH staining confirmed expression of AAV-hM4Di-mCherry in VTA dopamine neurons of DATcre+ mice. C, Ex vivo recordings confirmed CNO application to coronal VTA slices resulted in hyperpolarization of VTA TH+ve neurons in DATcre+ mice that had been previously injected with AAV-hM4Di-mCherry. D, E, Systemic CNO activation of AAV-hM4Di-mCherry before the salt appetite assay resulted in no significant effects on intake between genotypes (time × concentration × genotype, concentration × genotype, time × genotype all F < 1, all p > 0.5). Intake decreased over time (F(1.3,23.9) = 100.3, p < 0.001) with preference in concentration also changing over time (F(1.9,33.8) = 6.0, p < 0.001). F, Systemic CNO activation of AAV-hM4Di-mCherry in the VTA of DATcre+ (n = 10) and DATcre- (n = 12) mice resulted in a significant reduction in locomotor activity in DATcre+ mice, treatment × genotype F(1,18) = 8.5, p < 0.01 pairwise comparisons revealed significant effects only following CNO treatment, DATcre+ vs DATcre- p < 0.005. There was a significant interaction between treatment and time, with locomotor activity continuing to reduce with time in the CNO DATcre+ group (time × treatment F(11,198) = 2.8, p < 0.005). However, this did not differ between genotypes (time × treatment × genotype F(11,198) = 1.1, p > 0.3; time × genotype F < 1, p > 0.7). Data represented as mean ± SEM.