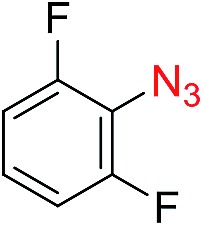
Table 2. Amidation of α-unsubstituted aldehydes a .
aProtocol: to a solution of azide 3 (1 mmol) and t-BuOK (2 equiv.) in THF/t-BuOH (1 mL/0.5 mL) under vigorous stirring, aldehyde 1 (4 mmol) in THF (0.5 mL) was added dropwise. After the reaction was completed (1–5 minutes), the solution was quenched with 1.5 M aq. AcOH (2 mL).
bIsolated yield.
c t-BuOK (1.2 equiv.), the volume of solvent was doubled.