Abstract
Background and aim
Gut microbiota may contribute to regulate colonic motility, which is involved in the etiology of constipation. Fecal microbiota transplantation (FMT) has been demonstrated to restore intestinal homeostasis. The aim of this study was to evaluate the clinical outcomes and prognostic factors of FMT for the treatment of slow transit constipation (STC).
Methods
Fifty-two patients with STC received standardized FMT and were followed up for 6 months. Bowel habit, colonic transit time, constipation-related symptoms (PAC-SYM score), quality of life (PAC-QOL score), treatment satisfaction scores and adverse events were monitored. The primary efficacy endpoint was the proportion of patients having on average three or more complete spontaneous bowel movements (CSBMs) per week.
Results
The primary efficacy endpoint was achieved in 50.0%, 38.5% and 32.7% of patients over week intervals 3–4, 9–12 and 21–24, respectively (P < 0.01 for all comparisons). Significant improvements were also observed in other bowel movement assessments, colonic transit time, constipation-related symptoms and quality of life; but all improvements diminished at weeks 12 and 24. Incompleteness of evacuation served as the only factor associated with efficacy. No serious treatment-related adverse events were observed.
Conclusion
This study suggested FMT was effective and safe for STC, while a late loss of efficacy was also observed. A lower degree of sensation of incompleteness predicted a better outcome.
Keywords: Fecal microbiota transplantation, slow transit constipation, gut microbiota, colonic motility, prognostic factors
Introduction
Chronic constipation is a common functional disorder, affecting 16% of adults and specifically 33.5% of the elderly worldwide [1]. Although chronic constipation is rarely associated with life-threatening or disabling problems, it does severely jeopardize the quality of life and represents a considerable health care burden because of the high prevalence and long-term duration [2,3]. The management of constipation remains challenging [4,5]. In order to alleviate symptoms, laxatives and prokinetic agents are commonly used [6]. Owing to insufficient efficacy, inconsistent symptom response and concerns about adverse effects, nearly half (47%) of patients are not completely satisfied with such treatments in a long-term survey [7].
Decreased colonic motility is an important pathophysiological mechanism of chronic constipation, especially slow transit constipation (STC). Recently, there have been studies suggesting that gut microbiota may be involved in the etiology of constipation. Imbalance in stool microbiota composition has been described in patients with constipation [8]. The gut microbiota and its metabolites, such as short-chain fatty acids and methane, show a direct modulation on gastrointestinal (GI) motility in both rodents and constipated patients [9–11]. Methods to modify the GI flora have drawn much attention for the management of chronic constipation [12]. However, trials of probiotics, prebiotics or synbiotics have showed uncertain results in constipated patients [13]. Using 16S rRNA sequencing, Zhu et al. found that the alterations in fecal microbiome of constipated patients were primarily decreased Prevotella and increased Firmicutes, but not the conventional probiotic genera Lactobacillus and Bifidobacteria [8]. Recently, Parthasarathy et al. reported similar results in both fecal and mucosal microbiota in chronic constipation [14]. This might explain why the traditional probiotics are ineffective in treatment for constipation. Instead, we hypothesized that reshaping the gut microbiome with fecal microbiota transplantation (FMT) might restore the abundance of ‘beneficial microflora’ in the colon and therefore promote colonic motility in constipated patients [12,15].
Our previous pilot studies suggested FMT had the potential to improve symptoms in patients with STC [16,17]. Here, we aimed to systematically evaluate the clinical outcomes and prognostic factors of FMT for STC in a larger prospective study with long-term follow-up.
Patients and methods
This is an open-label, single-group study conducted at Jinling Hospital, Medical School of Nanjing University. The study was approved by Ethical Committee of the hospital, and registered in the ClinicalTrials.gov (NCT02301221). All participants provided written informed consent.
Eligibility of patients
Inclusion criteria: chronic constipation according to Rome III criteria with two or fewer complete spontaneous bowel movements (CSBMs) per week for a minimum of 6 months [18]; age ≥18 years; body mass index (BMI): 18.5–25 kg/m2; slow colonic transit with colonic transit time >48 hours confirmed by colonic transit test [19]; normal anorectal manometry with no evidence of dyssynergia and confirmed ability to expel rectal balloon [4,5]; no radiographic evidence of functional (i.e. pelvic floor dyssynergia) or anatomical (i.e. significant rectocele and intussusception) impediment to the expulsion of the radio-opaque contrast [4,5]; disease duration >1 year; traditional treatment with dietary modification, laxatives and biofeedback tried over the past 6 months without success.
Exclusion criteria: megacolon or megarectum; secondary constipation (i.e. drugs, endocrine, metabolic, neurologic or psychologic disorders); diseases or therapies affecting intestinal motility or microbiota; previous abdominal, proctological or perianal surgery, except cholecystectomy, appendicectomy, tubal ligation and cesarean section; constipation-predominant irritable bowel syndrome (IBS-C) or functional abdominal pain syndrome according to Rome III criteria [18]; pregnant or lactating women; usage of probiotics, prebiotics and/or synbiotics within 1 month; usage of antibiotics and/or proton pump inhibitors (PPIs), smoking and/or alcohol addiction within 3 months.
Donor screening
Donors were healthy unrelated adults aged 18–30 years old, with a normal BMI of 18.5–25 kg/m2, good habits of eating and evacuation, and no recent medications or travels. After passing the American Association of Blood Banks donor questionnaire [20], candidates underwent screening tests for hepatitis A, B and C, HIV, Syphilis and Treponema Pallidum, and enteric pathogens including Clostridium difficile, Enterohemorrhagic Escherichia coli, Salmonella, Shigella, Yersinia, Campylobacter and parasites. Within 1 week before donation, the donors were asked to refrain from common food allergens. The questionnaire and laboratory screening were repeated every 3 months.
Preparation of fecal suspension
Approximately 100 grams of fresh stool was immediately homogenized in 500 milliliters of 0.9% sterile saline with a household blender. The slurry was then passed through 1.5, 0.6, 0.3 and 0.15 millimeter stainless steel sieves [21]. The filtered suspension was centrifuged at 5,000 grams for 15 minutes under 4°C and resuspended to 300 milliliters of saline [21]. Sterile glycerol was added with a final concentration of 10%. The suspension was repackaged and stored at –80°C until usage. Before usage, the preparation was thawed at 37°C. The maximal storage was 4 weeks.
FMT procedures
Eligible patients entered a 2-week run-in period. Patients having an average of three or more CSBMs per week during the run-in period were excluded. Oral vancomycin (500 milligrams two times per day) was given for three consecutive days, during which patients having a significant improvement on defecation were excluded. Bowel lavage with 2 liters of Macrogol solution was performed on the last day of antibiotic treatment. The next day, fecal suspension (100 millimeters once per day) was infused within 10 minutes through a nasoduodenal tube, which was positioned under endoscopy guidance in advance. The infusion was performed for 3 consecutive days and a full treatment of 300 millimeters of suspension contained sieved, concentrated material derived from approximately 100 grams of stool. After FMT, patients were followed up for 24 weeks, during which they were educated to maintain the previous diet, exercise and life habits.
Disallowed medication
During the follow-up, antibiotics were not permitted. The use of laxatives was conditional. If patients did not have a bowel movement for 3 or more consecutive days, they were permitted to take up to 20 grams of Macrogol 4000 powder (Forlax®, Ipsen, France). If ineffective, enema was used. Use of rescue medication was documented.
Bowel habit assessments
Patients kept daily diaries about times of bowel movements each day, stool consistency, degree of straining severity during defecation, degree of sensation of incompleteness of evacuation and the rescue medication when used. Stool consistency was assessed according to the Bristol Stool Form Scale (BSFS) [22]. A score of 3, 4 or 5 was defined as normal. Degree of straining severity and incompleteness of evacuation were assessed using a five-point ordinal scale [23,24], where 1 indicates none, 2 mild, 3 moderate, 4 severe and 5 very severe.
The primary efficacy endpoint was the proportion of patients having on average three or more CSBMs per week. Patients achieving the primary efficacy endpoint were defined as responders. The secondary efficacy endpoint was the proportion of patients with an average increase of one or more CSBMs per week compared with baseline. Other endpoints were the average number of CSBMs and spontaneous bowel movements (SBMs) per week; the percentage of bowel movements (BMs) with normal consistency, none or mild straining and none or mild incompleteness and the average number of days with laxative use (polyethylene glycol or enema) per week. All these data were evaluated over the 2-week run-in period and over the week intervals 3–4, 9–12 and 21–24.
Colonic transit time measurements
Colonic transit time was measured at baseline and at weeks 4, 12 and 24 with the Metcalf method [9]. Briefly, a capsule containing radio-opaque markers was taken at 9:00 a.m. every day for 6 consecutive days. A single abdominal X-ray was taken on the day after the administration of final capsule. Colonic transit time was calculated based on the number of markers detained in the colon.
Patient self-assessments
Constipation-related symptoms were evaluated using the validated Patient Assessment of Constipation Symptoms (PAC-SYM) questionnaire [25]. A total of 12 symptoms were grouped into three subscales: stool, abdominal and rectal symptoms. Quality of life was evaluated with the validated Patient Assessment of Constipation Quality of Life (PAC-QOL) self-report questionnaire [26]. Twenty-eight items were grouped into four subscales: physical discomfort, psychosocial discomfort, worries and concerns, and satisfaction. For each item of PAC-SYM or PAC-QOL questionnaire, scores range from 0 (not at all) to 4 (all the time), with lower scores indicating a better result. An improvement (reduction) of ≥1 point was regarded as clinically significant [25,26]. Treatment satisfaction was evaluated with the use of a five-point ordinal scale, ranging from 1 (not at all) to 5 (extremely) [23,24]. All these questionnaires were completed at baseline and at weeks 4, 12 and 24.
Safety assessments
During suspension infusion and follow-up, adverse events were recorded. If there was a sudden severe discomfort, patients were educated to make a phone call to investigators immediately.
Statistical analyses
Continuous data were presented as mean ± standard deviation, while categorical data were presented as number (%). Statistical analysis was performed with repeated measures ANOVA for continuous variables and Pearson’s chi-square test for categorical variables. Univariate analysis was performed using independent-samples t-test and Pearson’s chi-square test as appropriate, and factors with a meaningful univariate probability (P < 0.1) were included in the multivariate analysis using a logistic regression model with mixed effects. Analyses were performed with SPSS 22.0 software. A two-tailed P < 0.05 was considered as statistically significant.
Results
From March 2015 through March 2016, 60 patients were enrolled, of whom three were excluded prior to FMT and five withdrew during follow-up. Thus, 52 patients were included for analyses. The baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics
| Characteristics | All (n = 52) |
|---|---|
| Female | 34 (65.4) |
| Age, years | 48.9 ± 10.9 |
| BMI, kg/m2 | 22.8 ± 1.0 |
| Disease duration, years | 9.9 ± 7.1 |
| CSBMs, per week | 0.6 ± 0.5 |
| 0 | 18 (34.0) |
| >0 to ≤1 | 26 (50.9) |
| >1 to <3 | 8 (15.1) |
| Assessment of efficacy of previous treatment | |
| Adequate | 20 (38.5) |
| Inadequate | 32 (61.5) |
CSBMs, complete spontaneous bowel movements. Continuous data are presented as mean ± standard deviation, while categorical data are presented as number (%).
BMs
After FMT, the percentage of patients achieving primary efficacy endpoint (at least three CSBMs per week) over the week intervals 3–4, 9–12 and 21–24 increased to 50.0%, 38.5% and 32.7%, respectively (P < 0.01 for all comparisons with baseline) (Table 2). The proportion dropped as time went by, although the decrease was not significant compared with week interval 3–4.
Table 2.
Bowel habit assessments (n = 52)
| Endpoint | Run-in period | Follow-up |
||
|---|---|---|---|---|
| Weeks 3–4 | Weeks 9–12 | Weeks 21–24 | ||
| Mean CSBMs/week of ≥3 | 0 (0) | 26 (50.0)** | 20 (38.5)** | 17 (32.7)** |
| Increase of ≥1 CSBM/week | – | 38 (73.1) | 31 (59.6) | 23 (44.2)†† |
| CSBMs/week | 0.6 ± 0.5 | 2.8 ± 1.5** | 2.4 ± 1.4**†† | 1.8 ± 1.6**†† |
| SBMs/week | 2.1 ± 0.7 | 5.0 ± 1.4** | 4.6 ± 1.6**†† | 3.2 ± 1.4**†† |
| Percent of BMs with normal consistency (score = 3, 4 or 5) | 25.4 ± 20.2 | 52.1 ± 19.9** | 42.0 ± 21.0**†† | 35.8 ± 26.3*†† |
| Percent of BMs with none or mild straining (score = 1 or 2) | 19.2 ± 15.6 | 40.2 ± 23.6** | 34.1 ± 23.3**†† | 29.6 ± 25.0**†† |
| Percent of BMs with none or mild incompleteness (score = 1 or 2) | 37.8 ± 30.7 | 51.1 ± 18.1** | 47.0 ± 17.5**†† | 44.6 ± 28.5**† |
| Number of days with laxative use or enema/week | 1.0 ± 0.4 | 0.2 ± 0.4** | 0.4 ± 0.6**†† | 0.6 ± 0.6**†† |
BMs, bowel movements; SBMs, spontaneous bowel movements; CSBMs, complete spontaneous bowel movements. Continuous data are presented as mean ± standard deviation, while categorical data are presented as number (%). *P < 0.05, **P < 0.01 for the comparison with baseline; †P < 0.05, ††P < 0.01 for the comparison with week interval 3–4.
An average increase of one or more CSBMs per week compared with baseline was achieved in 73.1%, 59.6% and 44.2% of patients over week intervals 3–4, 9–12 and 21–24, respectively (Table 2). Meanwhile, the number of CSBMs and SBMs per week increased from a mean of 0.6 and 2.1 at baseline to 2.8 and 5.0, respectively, at week interval 3–4 (P < 0.01 for both comparisons with baseline), then decreased to 1.8 and 3.2 at week interval 21–24, respectively (P < 0.01 for both comparisons with week interval 3–4) (Table 2). Similar dynamic changes were observed for other endpoints, which were summarized in Table 2. Over the follow-up, it was noteworthy that all improvements diminished at weeks 12 and 24.
Colonic transit time
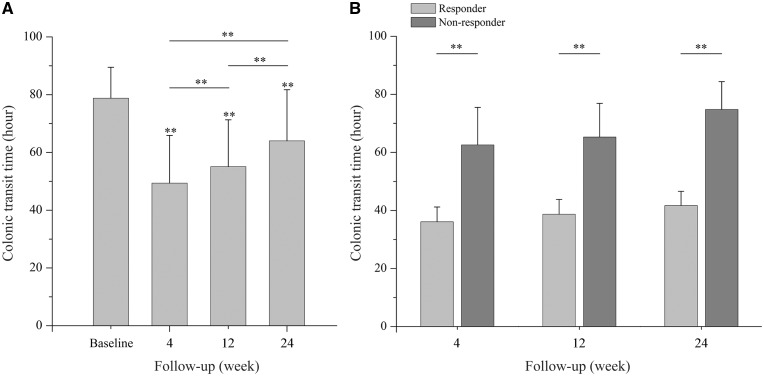
Compared with a mean of 78.8 hours at baseline, colonic transit time decreased significantly to 49.4, 55.1 and 64.0 hours at weeks 4, 12 and 24, respectively (P < 0.01 for all comparisons) (Figure 1a). However, when compared with week 4, colonic transit time rebounded significantly even at week 12 (P < 0.01). Patients were then divided into responders and non-responders according to whether achieving the primary efficacy endpoint at each time point. Mean colonic transit time at weeks 4, 12 and 24 were all significantly shorter in responders than non-responders (P < 0.01 for all comparisons) (Figure 1b).
Figure 1.
Changes of colonic transit time. (A) Mean colonic transit time in all patients at baseline and weeks 4, 12 and 24. (B) Mean colonic transit time in responders and non-responders at weeks 4, 12 and 24. *P < 0.05; **P < 0.01.
Patient self-assessments
Compared with baseline, the PAC-SYM score and PAC-QOL score decreased significantly at each time point (P < 0.01 for all comparisons). The proportion of patients with an improvement of one or more points on the PAC-SYM score reached 55.8%, 46.2% and 36.5% at weeks 4, 12 and 24, respectively, while the proportion for the PAC-QOL score arrived at 59.6%, 48.1% and 38.5%, respectively (Table 3). Although the reduction in all these scores remained significant at week 24 compared with baseline, significant increase of scores was observed compared to week 4. Over 6 months, the proportions of patients satisfied with treatment (treatment satisfaction score = 4 or 5) ranged from 63.5% at week 4 to 40.4% at week 24 (Table 3).
Table 3.
Patient self-assessments (n = 52)
| Score | Baseline | Follow-up |
||
|---|---|---|---|---|
| Week 4 | Week 12 | Week 24 | ||
| PAC-SYM score | 2.0 ± 0.2 | 1.2 ± 0.6** | 1.4 ± 0.6**†† | 1.6 ± 0.6**†† |
| Number of patients with improvement ≥1 PAC-SYM score | – | 29 (55.8) | 24 (46.2) | 19 (36.5)† |
| PAC-QOL score | 2.0 ± 0.2 | 1.1 ± 0.6** | 1.4 ± 0.6**†† | 1.6 ± 0.6**†† |
| Number of patients with improvement ≥1 PAC-QOL score | – | 31 (59.6) | 25 (48.1) | 20 (38.5)† |
| Number of patients satisfied with treatment (score = 4 or 5) | – | 33 (63.5) | 28 (53.8) | 21 (40.4)† |
PAC-SYM, Patient Assessment of Constipation Symptoms; PAC-QOL, Patient Assessment of Constipation Quality of Life. Continuous data are presented as mean ± standard deviation, while categorical data are presented as number (%). *P < 0.05, **P < 0.01 for the comparison with baseline; †P < 0.05, ††P < 0.01 for the comparison with week 4.
Prognostic factors for responses to FMT
The analyses of clinical factors for responses were performed according to the efficacy evaluated at week 4. Patients were divided into responders (n = 26) and non-responders (n = 26). In univariate analysis, age, disease duration, CSBMs per week, percent of BMs with none or mild straining, percent of BMs with none or mild incompleteness, PAC-SYM score and PAC-QOL score at baseline had a meaningful contribution to response to FMT (P < 0.1). In multivariate analysis, percent of BMs with none or mild incompleteness at baseline served as the only variable associated with efficacy (P = 0.037) (Table 4).
Table 4.
Univariate and multivariate analysis for responses to fecal microbiota transplantation
| Characteristic | Responders (n = 26) | Non-responders (n = 26) | P-value in univariate analysis | P-value in multivariate analysis |
|---|---|---|---|---|
| Female | 16 (61.5) | 18 (69.2) | 0.560 | – |
| Age, years | 45.2 ± 11.5 | 52.5 ± 9.1 | 0.014 | 0.307 |
| BMI, kg/m2 | 22.9 ± 1.0 | 22.8 ± 1.1 | 0.537 | – |
| Disease duration, years | 8.0 ± 4.0 | 11.8 ± 9.0 | 0.058 | 0.366 |
| CSBMs/week | 0.7 ± 0.5 | 0.4 ± 0.5 | 0.091 | 0.910 |
| SBMs/week | 2.1 ± 0.7 | 2.1 ± 0.8 | >0.999 | – |
| Percent of BMs with normal consistency | 29.8 ± 21.5 | 20.9 ± 18.2 | 0.113 | – |
| Percent of BMs with none or mild straining | 23.5 ± 14.8 | 14.8 ± 15.6 | 0.045 | 0.291 |
| Percent of BMs with none or mild incompleteness | 58.7 ± 25.7 | 16.9 ± 18.8 | <0.001 | 0.037 |
| Number of days with laxative use or enema/week | 0.9 ± 0.4 | 1.0 ± 0.4 | 0.254 | – |
| Colonic transit time, hours | 78.2 ± 11.1 | 79.3 ± 10.6 | 0.702 | – |
| PAC-SYM score | 2.0 ± 0.1 | 2.1 ± 0.2 | 0.011 | 0.078 |
| PAC-QOL score | 2.0 ± 0.2 | 2.1 ± 0.2 | 0.043 | 0.116 |
BMs, bowel movements; SBMs, spontaneous bowel movements; CSBMs, complete spontaneous bowel movements; PAC-SYM, Patient Assessment of Constipation Symptoms; PAC-QOL, Patient Assessment of Constipation Quality of Life. Patients achieving the primary efficacy endpoint at week 4 were defined as responders. Continuous data are presented as mean ± standard deviation, while categorical data are presented as number (%).
Safety
No serious adverse events were observed. A detailed description of adverse events was included in Table 5. During days of infusion, the most common complications were borborygmi (19.2%), flatulence (15.4%) and nausea (13.5%). Diarrhea (11.5%) and abdominal pain (7.7%) were also reported. No patients reported fever and intolerant abdominal pain or cramps. During follow-up, flatulence (17.3%) was also the most frequent adverse event. Other adverse events were occasional diarrhea (7.7%), abdominal pain (3.8%), increased bloating (5.8%) and borborygmi (7.7%). Discomfort related to nasoduodenal tube, such as nausea, vomiting and nasopharyngitis, disappeared completely as the tube was removed.
Table 5.
Treatment-related adverse events (n = 52)
| Adverse event | On days of infusion | During follow-up |
|---|---|---|
| Fever | 0 (0) | 0 (0) |
| Diarrhea | 6 (11.5) | 4 (7.7) |
| Abdominal pain | 4 (7.7) | 2 (3.8) |
| Increased bloating | 0 (0) | 3 (5.8) |
| Borborygmi* | 10 (19.2) | 4 (7.7) |
| Flatulence | 8 (15.4) | 9 (17.3) |
| Nausea | 7 (13.5) | 0 (0) |
| Vomiting | 1 (1.9) | 0 (0) |
| Nasopharyngitis | 4 (7.7) | 0 (0) |
* No patients with borborygmi reported abdominal cramps. Categorical data were presented as number (%).
Discussion
This is a prospective study with long-term follow-up to comprehensively evaluate the efficacy and safety of FMT for STC. Compared with our previous study, this study involved more patients and we are more concerned about the long-term efficacy of FMT. As a result, we found that FMT increased BMs, decreased colonic transit time, and improved PAC-SYM scores and PAC-QOL scores. However, an obvious diminution of efficacy was also observed. The multivariate analysis showed a lower degree of sensation of incompleteness predicted a better clinical outcome.
Dysmotility is one of the most difficult situations of GI diseases. Gut microbiota acts as a promising target for constipation therapy due to its effects on gut motility [9–11]. Indigenous bacteria from the gut flora were demonstrated to regulate the metabolism of gut-derived hormones, such as glucagon-like peptide-1 (GLP-1) and serotonin [27,28]. They were also found to drive the crosstalk between muscularis macrophages and enteric neurons that controlled the pattern of smooth muscle contractions and the peristaltic activity of the colon [29]. However, the application of probiotics, prebiotics or synbiotics did not achieve satisfactory efficacy for chronic constipation, with the possible explanation that the traditional probiotics Lactobacillus and Bifidobacteria might not be the key microbiota for motility regulation [8,14]. The optimal method would be to screen for bacteria species that regulate colonic motility in constipated patients and develop the microflora ‘cocktail’ treatment. But, before that, the FMT might be an attractive option.
In this study, the proportion of patients achieving the primary efficacy endpoint could reach a maximum value of 50.0% at week interval 3–4, which was better than the result of prucalopride (33.8%) [23]. The primary endpoint of average three or more CSBMs per week was chosen, since it is considered as the low end of the range that defines normal bowel function [23]. It meant that half of the patients got rid of constipation at 4 weeks after FMT. Meanwhile, over two-thirds (73.1%) of the patients had an average increase of one or more CSBMs per week. The improvements were accompanied by the decline in colonic transit time, and a significant difference in colonic transit time between responders and non-responders was observed. These findings served as solid evidence for the hypothesis that the efficacy of FMT on constipation at least partly depended on its effects on colonic motility. The univariate and multivariate analysis of baseline characteristics between responders and non-responders found that the percent of BMs with none or mild incompleteness was the only factor associated with poor clinical outcome, which suggested FMT would be more suitable for patients with a lower degree of sensation of incompleteness.
It is worthwhile to note that, even for responders, an obvious diminution of efficacy was also observed, although definite improvements were still observed until the end of 24-week follow-up. Recently, Vandeputte et al. observed a strong association between stool consistency and gut microbiota richness and composition [30–32], while, Parthasarathy et al. found that the profile of the fecal microbiota, especially genera from Firmicutes (Faecalibacterium, Lactococcus and Roseburia) correlated with colonic transit [14]. The diminished efficacy might be due to the decreasing colonization of donor microbiota and predomination of the recipient bacteria over time. A dynamic evaluation of the gut microbiota after FMT may be helpful in verifying this presumption [33].
The main limitation of this study was the lack of a controlled group. This study was a further investigation with larger sample size and longer follow-up based on our previous pilot studies [16,17]. As the long-term effects of microbiota manipulation were still unclear, a 6-month follow-up was elected in this study. According to the available data regarding efficacy of placebo in previous randomized controlled trials (RCTs) [23,24], it would be difficult and inhumane for patients in the placebo group to complete the 6-month follow-up, considering the poor quality of life for constipated patients. A RCT with shorter follow-up is being conducted in our center (NCT02526849), in which the changes of gut microbiota will be also evaluated.
In conclusion, this study showed that FMT was effective and safe for STC. Patients with a lower degree of sensation of incompleteness could achieve a better outcome. However, an obvious diminution of efficacy was also observed, which called for regular supplements, such as frozen or freeze-dried FMT capsules [34,35]. Larger randomized controlled trials are needed to further assess the benefits and risks of FMT for constipation.
Acknowledgements
The study was supported by the National Natural Science Foundation of China (81670493) and the National Gastroenterology Research Project (2015BAI13B07).
Conflict of interest statement: none declared.
References
- 1. Lembo A, Camilleri M. Chronic constipation. New Engl J Med 2003;349:1360–8. [DOI] [PubMed] [Google Scholar]
- 2. Dennison C, Prasad M, Lloyd A, et al. The health-related quality of life and economic burden of constipation. Pharmacoeconomics 2005;23:461–76. [DOI] [PubMed] [Google Scholar]
- 3. Irvine EJ, Ferrazzi S, Pare P, et al. Health-related quality of life in functional GI disorders: focus on constipation and resource utilization. Am J Gastroenterol 2002;97:1986–93. [DOI] [PubMed] [Google Scholar]
- 4. Bharucha AE, Dorn SD, Lembo A, et al. American Gastroenterological Association medical position statement on constipation. Gastroenterology 2013;144:211–17. [DOI] [PubMed] [Google Scholar]
- 5. Bharucha AE, Pemberton JH, Locke III GR. American Gastroenterological Association technical review on constipation. Gastroenterology 2013;144:218–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ford AC, Suares NC. Effect of laxatives and pharmacological therapies in chronic idiopathic constipation: systematic review and meta-analysis. Gut 2011;60:209–18. [DOI] [PubMed] [Google Scholar]
- 7. Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharm Therap 2007;25:599–608. [DOI] [PubMed] [Google Scholar]
- 8. Zhu LX, Liu WS, Alkhouri R, et al. Structural changes in the gut microbiome of constipated patients. Physiol Genomics 2014;46:679–86. [DOI] [PubMed] [Google Scholar]
- 9. Kashyap PC, Marcobal A, Ursell LK, et al. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology 2013;144:967–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Soret R, Chevalier J, De Coppet P, et al. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 2010;138:1772–82. [DOI] [PubMed] [Google Scholar]
- 11. Attaluri A, Jackson M, Valestin J, et al. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am J Gastroenterol 2010;105:1407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pinn DM, Aroniadis OC, Brandt LJ. Is fecal microbiota transplantation (FMT) an effective treatment for patients with functional gastrointestinal disorders (FGID)? Neurogastroent Motil 2015;27:19–29. [DOI] [PubMed] [Google Scholar]
- 13. Ford AC, Quigley EMM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol 2014;109:1547–61. [DOI] [PubMed] [Google Scholar]
- 14. Parthasarathy G, Chen J, Chen XF, et al. Relationship between microbiota of the colonic mucosa vs feces and symptoms, colonic transit, and methane production in female patients with chronic constipation. Gastroenterology 2016;150:367–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andrews PJ, Barnes P, Borody TJ. Chronic constipation reversed by restoration of bowel floraA a case and and a hypothesis. Eur J Gastroen Hepat 1992;4:245–7. [Google Scholar]
- 16. Tian H, Ding C, Gong J, et al. Treatment of slow transit constipation with fecal microbiota transplantation: a pilot study. J Clin Gastroenterol 2016;50:865–70. [DOI] [PubMed] [Google Scholar]
- 17. Ge X, Tian H, Ding C, et al. Fecal microbiota transplantation in combination with soluble dietary fiber for treatment of slow transit constipation: a pilot study. Arch Med Res 2016;47:236–42. [DOI] [PubMed] [Google Scholar]
- 18. Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 2006;130:1377–90. [DOI] [PubMed] [Google Scholar]
- 19. Emmanuel A, Cools M, Vandeplassche L, et al. Prucalopride improves bowel function and colonic transit time in patients with chronic constipation: an integrated analysis. Am J Gastroenterol 2014;109:887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fridey JL, Townsend MJ, Kessler DA, et al. A question of clarity: redesigning the American Association of Blood Banks blood donor history questionnaire—a chronology and model for donor screening. Transfus Med Rev 2007;21:181–204. [DOI] [PubMed] [Google Scholar]
- 21. Hamilton MJ, Weingarden AR, Sadowsky MJ, et al. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol 2012;107:761–7. [DOI] [PubMed] [Google Scholar]
- 22. Heaton KW, Radvan J, Cripps H, et al. Defecation frequency and timing, and stool from in the general population: a prospective study. Gut 1992;33:818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Camilleri M, Kerstens R, Rykx A, et al. A placebo-controlled trial of prucalopride for severe chronic constipation. New Engl J Med 2008;358:2344–54. [DOI] [PubMed] [Google Scholar]
- 24. Tack J, van Outryve M, Beyens G, et al. Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives. Gut 2009;58:357–65. [DOI] [PubMed] [Google Scholar]
- 25. Frank L, Kleinman L, Farup C, et al. Psychometric validation of a constipation symptom assessment questionnaire. Scand J Gastroenterol 1999;34:870–7. [DOI] [PubMed] [Google Scholar]
- 26. Marquis P, De la Loge C, Dubois D, et al. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol 2005;40:540–51. [DOI] [PubMed] [Google Scholar]
- 27. Wichmann A, Allahyar A, Greiner TU, et al. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe 2013;14:582–90. [DOI] [PubMed] [Google Scholar]
- 28. Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015;161:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Muller PA, Koscsó B, Rajani GM, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 2014;158:300–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vandeputte D, Falony G, Vieira-Silva S, et al. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016;65:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tigchelaar EF, Bonder MJ, Jankipersadsing SA, et al. Gut microbiota composition associated with stool consistency. Gut 2016;65:540–2. [DOI] [PubMed] [Google Scholar]
- 32. Hadizadeh F, Walter S, Belheouane M, et al. Stool frequency is associated with gut microbiota composition. Gut 2017;66:559–60. [DOI] [PubMed] [Google Scholar]
- 33. Angelberger S, Reinisch W, Makristathis A, et al. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol 2013;108:1620–30. [DOI] [PubMed] [Google Scholar]
- 34. Youngster I, Russell GH, Pindar C, et al. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 2014;312:1772–8. [DOI] [PubMed] [Google Scholar]
- 35. Tian HL, Ding C, Gong JF, et al. Freeze-dried, capsulized fecal microbiota transplantation for relapsing Clostridium difficile infection. J Clin Gastroenterol 2015;49:537–8. [DOI] [PubMed] [Google Scholar]