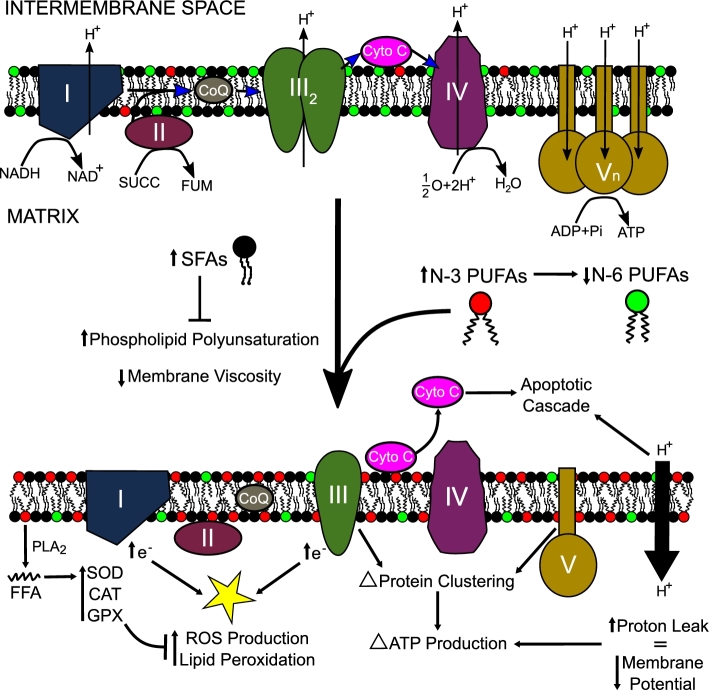
FIGURE 3.
Model depicting how dietary SFA, n–6 PUFA, and n–3 PUFA acyl chains target IMM structure-function. An increase in n–3 PUFA acyl chains within the IMM will replace the n–6 PUFA acyl chains and thereby influence microviscosity. This may cause the membrane to become “leaky” and allow for more proton leak back into the matrix. In addition, an increase in SFAs will decrease polyunsaturation and increase viscosity. n–3 PUFA incorporation into the membrane may also alter protein clustering and enzyme activity, which may alter the amount of ATP produced. Respiratory enzymes are influenced by the increase in n–3 PUFAs and may allow more electrons to escape during oxidative phosphorylation, which will lead to an increase in ROS production and peroxidation. However, n–3 PUFAs can also be cleaved from the membrane via PLA2 and increase the antioxidant capacity of the mitochondria. In addition, an increase in n–3 PUFAs may release cytochrome c, starting the apoptotic cascade as seen in some cancer models. Overall, FAs through the diet likely have a wide range of different roles within the IMM that require further investigation in both healthy and diseased states. CAT, catalase; CoQ, coenzyme Q; Cyto C, cytochrome c; FUM, fumarate; GPX, glutathione peroxidase; IMM, inner mitochondrial membrane; Pi, inorganic phosphate; PLA2, phospholipase A2; SOD, superoxide dismutase; SUCC, succinate.