Table 4.
Non-nucleotide Compounds Discussed in This Studyd
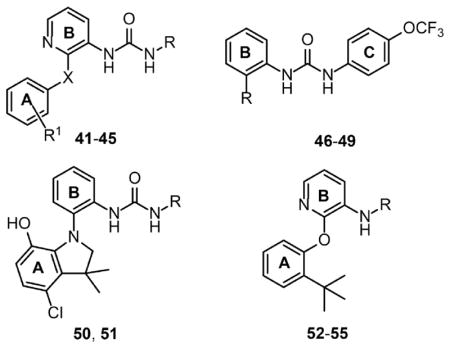
| |||||
|---|---|---|---|---|---|
| No. | R | R1 | X | Ki(nm)a | Ref. |
| 41c | 4-OCF3-Ph | 2-t-Bu | O | 6.0 ± 0.6 | 24 |
|
| |||||
| Substitutions on ring A | |||||
| 42 | 4-Cl-Ph | 3-CF3 | O | 69 ± 23 | 24 |
| 43 | CH2-Ph | 3-CF3 | O | >5000 | 24 |
| 44 | 2,4-di-F-Ph | 4-Me | O | >5000 | 24 |
|
| |||||
| Replacement of ether linker | |||||
| 45 | 4-Me-Ph | 2-t-Bu | NMe | >5000 | 24 |
| 46 |

|
8.7 ± 1.7 | 27 | ||
| 47 |

|
7.3 ± 0.6 | 27 | ||
| 48 |

|
830 ± 5 | 27 | ||
| 49 |

|
4.3 ± 2.6 | 27 | ||
| 50 |

|
b | 28 | ||
| 51 |

|
b | 28 | ||
|
| |||||
| Replacement of urea moiety | |||||
| 52 |

|
11 | 30 | ||
| 53 |

|
2500 | 30 | ||
| 54 |

|
45 | 30 | ||
| 55 |

|
>36,000 | 27 | ||
Compounds were tested at the hP2Y1R.
Binding Ki values were not reported for compounds 50 and 51, but their reported IC50 values in calcium mobilization determined using a fluorescent imaging plate reader (FLIPR) are 0.14 ± 0.04 and 0.39 ± 0.28 nM, respectively.
41, BPTU.
Compounds were previously reported and tested in binding at the P2Y1R.
