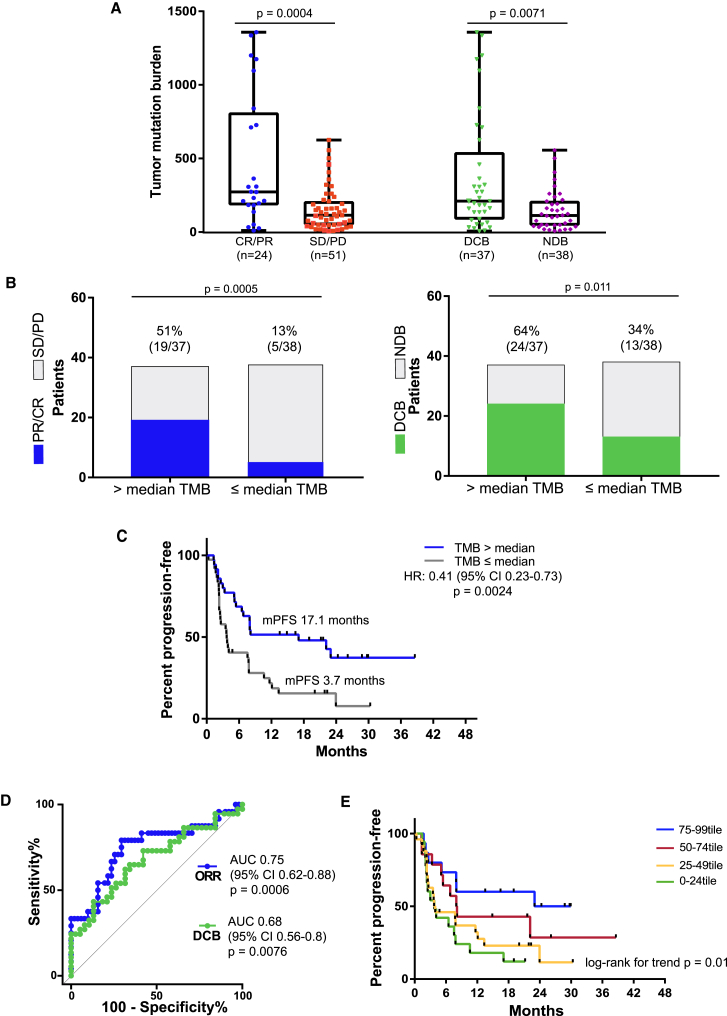
Figure 1.
TMB Correlates with Efficacy in Patients with NSCLC Treated with Nivolumab Plus Ipilimumab
(A) TMB in patients with complete response (CR)/partial response (PR) (n = 24, blue) versus stable disease (SD)/progressive disease (PD) (n = 51, red) (median 273 versus 114 mutations, Mann-Whitney p = 0.0004) and TMB in patients with DCB (green, n = 37) versus those with NDB (purple, n = 38) (median 210 versus 113 mutations, Mann-Whitney p = 0.0071). Medians, interquartile ranges, and minimum/maximum shown in boxplots.
(B) Objective response and durable clinical benefit in patients with high TMB (>median, 158 mutations) versus low TMB (≤median) (ORR 51% versus 13%, odds ratio 6.97 [95% confidence interval (CI) 2.19–19.0], Fisher's exact p = 0.0005; DCB 65% versus 34%, odds ratio 3.55 [95% CI 1.3–8.64], Fisher's exact p = 0.011). Proportion of CR/PR or DCB, respectively, are colored on histograms with rate (n/N) shown above each bar.
(C) PFS in patients with high TMB versus low TMB (median 17.1 versus 3.7 months, Mantel-Haenszel hazard ratio 0.41 [95% CI 0.23–0.73], log rank p = 0.0024).
(D) Receiver operating characteristic (ROC) curves for correlation of TMB with objective response (CR/PR; blue line) (AUC 0.75 [95% CI 0.62–0.88], p = 0.0006) and DCB (green line) (AUC 0.68 [95% CI 0.56–0.8], p = 0.0076).
(E) PFS in cohorts of patients defined by quartiles of TMB percentile rank among NSCLC tumors profiled by TCGA (log rank for trend p = 0.01).