Abstract
Teleost fish are known to express two isoforms of P450 aromatase, a key enzyme for estrogen synthesis. One of the isoforms, brain aromatase (AroB), cyp19a1b, is highly expressed during early development of zebrafish, thereby suggesting its role in brain development. On the other hand, early development of serotonergic neuron, one of the major monoamine neurons, is considered to play an important role in neurogenesis. Therefore, in this study, we investigated the role of AroB in development of serotonergic neuron by testing the effects of (1) estradiol (E2) exposure and (2) morpholino (MO)-mediated AroB knockdown. When embryos were exposed to E2, the effects were biphasic. The low dose of E2 (0.005 µM) significantly increased serotonin (5-HT) positive area at 48 hour post-fertilization (hpf) detected by immunohistochemistry and relative mRNA levels of tryptophan hydroxylase isoforms (tph1a, tph1b, and tph2) at 96 hpf measured by semi-quantitative PCR. To test the effects on serotonin transmission, heart rate and thigmotaxis, an indicator of anxiety, were analyzed. The low dose also significantly increased heart rate at 48 hpf and decreased thigmotaxis. The high dose of E2 (1 µM) exhibited opposite effects in all parameters. The effects of both low and high doses were reversed by addition of estrogen receptor (ER) blocker, ICI 182,780, thereby suggesting that the effects were mediated through ER. When AroB MO was injected to fertilized eggs, 5-HT-positive area was significantly decreased, while the significant decrease in relative tph mRNA levels was found only with tph2 but not with two other isoforms. AroB MO also decreased heart rate and increased thigmotaxis. All the effects were rescued by co-injection with AroB mRNA and by exposure to E2. Taken together, this study demonstrates the role of brain aromatase in development of serotonergic neuron in zebrafish embryos and larvae, implying that brain-formed estrogen is an important factor to sustain early development of serotonergic neuron.
Keywords: estradiol, brain aromatase, biphasic manner, serotonergic neuron, zebrafish, early development
Introduction
Biosynthesis of estrogen is catalyzed by the action of cytochrome P450 aromatase, a product of cyp19a1 gene (1, 2). Contrary to mammals, zebrafish and many other teleosts have two isoforms of aromatase gene, cyp19a1a and cyp19a1b, encoding ovarian and brain aromatase, respectively (3, 4), and their predominant expression in respective tissues indicates differential regulation and functions. Fish brain is characterized by having much higher aromatase expression in brain compared to mammals (5). At the same time, fish brain has been reported to exhibit elevated neuroregenerative capacity compared to mammals (6–8). Widespread proliferation zones are detected in zebrafish brain (6, 9), while only limited areas such as subependymal and subgranular zones exhibit proliferation in mammals (7). Such high neurogenic activity in teleost fish may be attributed to increased synthesis of estrogen due to the elevated expression of brain aromatase. Indeed, expression of brain aromatase is localized in radial glial cells (RGCs), which differentiate into neurons and other glial cells contributing to adult neurogenesis as well as developmental neurogenesis (10–12). Developmental studies in zebrafish show that expression of brain aromatase in embryos increases rapidly after 12 hour post-fertilization (hpf), and is regulated by positive feedback loop through its own product, estrogen, acting on estrogen response element of cyp19a1b (3, 13, 14). Therefore, the zebrafish model expressing elevated levels of brain aromatase in early development is suitable to investigate the functional significance of aromatase and neural estrogen in developing brain.
Serotonin (5-HT), a neurotransmitter produced by multiple enzymatic steps including a rate-limiting action of tryptophan hydroxylase (TPH), plays a major role in a number of physiological processes and pathological conditions, such as depression (15, 16), stress (15, 17), cardiac function (18), reward seeking behavior (19), and anxiety (15, 20). In addition, serotonergic neuron is known to be involved in neurogenic activities (21). It has been reported that 5-HT is critically involved in the brain plasticity, neural trafficking, synapse formation, and network construction during development (22, 23). Serotonergic neurons in raphe nuclei extend their axons to the forebrain possibly modulating the differentiation of neuronal progenitors (24). Early ontogeny of serotonergic system may further suggest its role in brain development (25). Raphe 5-HT populations in human brain are considered as the earliest to be identified (24, 26).
Serotonergic neurons in mammalian brain are localized mainly in raphe nuclei of brain stem, which project into accumbens, hypothalamus, substantia nigra, and periaqueductal gray (22, 23). On the other hand, 5-HT-positive cell bodies are detected mainly in three populations in adult fish brain: pretectal area, posterior tuberculum/hypothalamus, and raphe (27, 28). Interestingly, distributions of serotonergic populations and their fibers overlap with highly proliferative areas of fish brain, which may indicate serotonergic regulation in adult neurogenesis in fish (27). In adult zebrafish, serotonin has been shown to promote regeneration of motor neurons by acting on progenitor cells (29).
It is well documented that serotonergic neuron is one of the targets of estrogen in mammals (30, 31). In macaques, estrogen increases gene expression and protein contents for TPH (32), and decreases gene expression of the serotonin reuptake transporter and the 5HT1A autoreceptor (33, 34). In mammals, both ERα and ERβ are expressed in 5-HT neurons with differential distributions depending on species and sex (35–37). ERβ has been shown to regulate tph2 expression in serotonergic neurons (38, 39). Similarly in teleost fish, effects of ovarian steroids on serotonin system have been reported in some species. In tilapia, the response of 5-HT content in brain to E2 treatment was dependent on developmental stages. Treatment between days 7 and 10 posthatching decreased 5-HT content, while the treatment at later stages increased it (40). Similar result was obtained in Japanese sea bass, which shows a significant decrease in brain 5-HT content in fingerlings after E2 treatment, while the content increased in juvenile group (41). Indeed, overlapping distributions of ER with raphe 5-HT innervation in telencephalon and diencephalon of adult zebrafish brain implies close association of ER and serotonergic neurons (27, 42). It has been reported that ERβ exhibits broad distribution along the brain ventricles of telencephalon and diencephalon in adult zebrafish (43), though co-localization of ER in serotonergic neurons has yet to be documented in fish.
Therefore, in this study, we tested the hypothesis that brain aromatase modulates serotonergic neuron in early development of zebrafish. In order to elucidate a possible role of brain-formed estrogen, we first examined the effects of exogenous E2 and then MO-mediated knockdown of brain aromatase on parameters such as 5-HT contents, relative tph expression levels, heart rate, and thigmotaxis in zebrafish embryos and larvae.
Materials and Methods
Fish Maintenance and Embryo Culture
Adult zebrafish (Danio rerio) were obtained from the local pet shop and reared in a 60-L tank. Water temperature was maintained at 26–30°C, and the light regime was 14 h of light starting at 10:00 followed by 10 h of dark. Fish were fed with TetraMin (Tetra Japan Inc.) twice a day. Fertilized eggs were collected within 15 min after fertilization and washed in embryo medium (EM) (0.004% CaCl2, 0.163% MgSO4, 0.1% NaCl, and 0.003% KCl) to remove debris. Embryos were transferred to a 6-well plastic plate (30 embryos in 8 mL of EM per well), and incubated at 28 ± 0.5°C. The medium was changed daily. All experimental procedures and maintenance of fish were conducted in accordance with the Guide for Care and Use of Laboratory Animals published by the US National Institutes of Health.
Exposure Experiments
Stock solutions of 17β-estradiol (E2) (Sigma-Aldrich) at 10 mM, ICI 182,780 (ICI) (Tocris Bioscience) at 10 mM, and dexamethasone (DEX) (Wako) at 100 mM were prepared in dimethyl sulfoxide (DMSO), and diluted with EM to the final concentrations indicated in the experiments. Quipazine maleate salt (Q) (Sigma-Aldrich) and fluoxetine hydrochloride (FLX) (Wako) were dissolved in ethanol at 100 and 10 mM, respectively, which were further diluted with EM to the final concentrations used in the experiments. Control embryos were cultured in 0.1% DMSO or ethanol. Exposure started at 2 hpf and continued till embryos and larvae were subjected to the assays. The media were changed daily.
Morpholino (MO) Microinjection
Morpholino antisense oligos were purchased from Gene Tool. MO sequences are shown in Table 1. MOs were dissolved in distilled water to 50 mg/mL and stored at −20°C. Before injection, MO solution was heated at 65°C for 5 min and further diluted to the working concentrations (2.5 and 5 ng/nL) with deionized H2O containing rhodamine B (Wako). The final concentration of rhodamine B was 0.08%. MO was injected into embryos at one to four cell stages using a glass microcapillary injection needle attached to the automatic nanoliter injector (Drummond Scientific). Injection volume was set at 2.3 nL per embryo. After the injection, embryos were observed under the fluorescence microscope (Leica M165 FC), and embryos that did not exhibit red fluorescence were discarded. To examine the effect of MO-mediated AroB knockdown, AroB MO designed to block translation was injected (2.5 and 5 ng/nL). Uninjected embryos (C), embryos injected with 5 ng/nL of standard control MO (Std MO), and inverted AroB MO (InvB MO) served as control groups. MO to block translation of cyp19a1a, ovarian aromatase (AroA MO) was also tested for 5-HT immunohistochemistry. As it has been reported that MO injection will cause off-target effect such as apoptosis through activation of p53 gene (44, 45), MO to block translation of p53 (p53 MO) at 2 ng/nL was co-injected with AroB MO at 5 ng/nL.
Table 1.
Morpholino (MO) sequences.
| Name | Sequence | Reference |
|---|---|---|
| AroA MO (ovarian aromatase MO) | GGAGCAGATCACCTGCCATAAGAAC | This paper |
| Genebank Acc. No.: AF226620 | ||
| InvA MO (inverted ovarian aromatase MO) | CAAGAATACCGTCCACTAGACGAGG | This paper |
| AroB MO (brain aromatase MO) | ATCCTTTACCACATGCTCCATCATC | This paper |
| Genebank Acc. No.: AF226619 | ||
| InvB MO (inverted brain aromatase MO) | CTACTACCTCGTACACCATTTCCTA | This paper |
| Std MO (standard control MO) | CCTCTTACCTCAGTTACAATTTATA | Gene tools |
| p53 MO | GCGCCATTGCTTTGCAAGAATTG | (44, 45) |
| Genebank Acc. No.: NM 131327 | ||
For rescue experiments, the AroB mRNA (30 pg/nL) was co-injected with AroB MO (5 ng/nL). The full length AroB cDNA was obtained by One Step PrimeScript RT-PCR Kit (Takara) using total RNA from 7-dpf zebrafish larvae and AroB primers (Table 2). Amplified products were purified with NucleoSpin Gel and PCR Clean-up (Machery-Nagel) and subcloned into pGEM-T Easy Vector (Promega). Nucleotide sequences and orientation of the inserts were verified by DNA sequencing analysis carried out using BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) and ABI 3130 xl genetic analyzer (Applied Biosystems). Plasmid DNA was linearized with SalI and the full length AroB mRNA was transcribed in vitro by MAXIscript T7 Kit (Ambion).
Table 2.
PCR primers and conditions.
| Gene | Primer sequence (5′ → 3′) | Size of PCR product (bp) | Amplification profile |
|---|---|---|---|
| tph1a | F: TTCAAGGACAATGTCTATCG | 214 | 94°C—30 s |
| R: GGGAGTCGCAGTGTTTGATG | 55°C—30 s | ||
| Genebank Acc. No.: AF548566 (46) | 72°C—60 s (35 cycles) | ||
| tph1b | F: TACCTGCAGAACCTGCCTCT | 430 | 94°C—30 s |
| R: AGAGAAGACCAGCCCCGTAT | 55°C—30 s | ||
| Genebank Acc. No.: BC154120 (46) | 72°C—60 s (35 cycles) | ||
| tph2 | F: GTGTGAACTCCAAAGCAGCA | 684 | 94°C—30 s |
| R: TGGTATTCCTTCCCCATCTG | 55°C—30 s | ||
| Genebank Acc. No.: AB125219 (46) | 72°C—60 s (35 cycles) | ||
| cyp19a1b (AroB) | F: TTAAAGAGGTGTGTCTGTATGTGAGGTG | 1,435 | 42°C—10 min |
| R: GGAATTTACTCTGTGCGCCTTTAAATGT | 94°C—30 s | ||
| Genebank Acc. No: BC076104 | 60°C—15 s | ||
| 72°C—90 s (40 cycles) | |||
| β-Actin | F: GGTATGGGACAGAAAGACAG | 330 | 94°C—30 s |
| R: AGAGTCCATCACGATACCAG | 58°C—30 s | ||
| Genebank Acc. No: AF025305 (3) | 72°C—60 s (34 cycles) | ||
Western Blot and Dot Blot Analysis
The antiserum to brain aromatase was produced in a rabbit against the synthetic peptide, CNSNGETADNRTSKE of zebrafish AroB (Sigma-Genosys). This peptide sequence has been used to raise the specific antibody as previously described (47). To confirm the specificity of the antiserum, Western blot of brain extract was conducted. Adult female zebrafish were exposed to E2 (5 and 25 ng/L) or vehicle alone (0.00025% DMSO) for 24 h (three fish per group). Brains were pooled and homogenized in HBST buffer (100 mM NaCl, 10 mM HEPES, 0.5% Triton-X 100, 0.01% TPCK, and 0.01% TLCK). After centrifugation at 10,000 g for 10 min, protein concentrations in supernatant were measured using BCA protein assay kit (Thermo Scientific). Extracts (30 µg protein/sample) were separated on 12.5% SDS-PAGE and transferred to a PVDF membrane. Precision Plus Protein Unstained Standards (Bio-Rad) were used for size reference. After blocking by 1% skim milk in PBS for 1 h, the membrane was incubated with the AroB antiserum (1:500) for 2 h, and then with the secondary antibody conjugated with alkaline phosphatase (AP) (Abcam) (1:1,000) for 1 h. After washing, the membrane was incubated in AP buffer (0.1 M Tris–HCl, pH 9.5, 0.1 M NaCl, 1 M MgCl) for 1 min. Signals were developed for 2–3 min in BCIP/NBT substrate (Roche) diluted at 1:50 in AP buffer, and the reaction was stopped by 0.5 M EDTA. All the incubation steps were done at RT. The antiserum to ovarian aromatase was raised in a rabbit using a synthetic peptide, CKPDVYFRLDWLHKKHKRD of zebrafish AroA (Sigma-Genosys). Similarly, Western blot with the antiserum (1:500) was performed using the ovarian extract prepared with HBST buffer from three adult fish (30 μg/lane).
To examine the effect of MO-mediated AroB knockdown, dot blot analysis using 120 larvae at 6 dpf collected from 4 separate MO injection experiments were pooled and extracted similarly as described for the brain extract. Extracts containing 40 µg protein (3 µL) were spotted onto nitrocellulose membrane (GVS Life Science). The membrane was treated similarly as in Western blot except for the concentration of the secondary antibody at 1:2,000. Density of the blots were analyzed with NIH ImageJ software. Blots of embryo extracts treated with the pre-immunized rabbit serum were used as a negative control to subtract from the density obtained with the AroB antiserum. No changes were observed among controls (uninjected, standard control MO, and inverted AroB MO) (data not shown). The effect of MO-mediated AroA knockdown was also examined by dot blot analysis. Briefly, pooled 120 embryos at 2 dpf collected from 5 separate embryo cohorts were extracted. Extracts containing 30 µg protein (3 µL) were spotted onto nitrocellulose membrane and subjected to immunostaining using AroA antiserum at 1:500. No changes were observed among controls including inverted AroA MO (data not shown).
5-HT Immunohistochemistry
Whole-mount immunohistochemistry for 5-HT was carried out according to the previous studies (48, 49). 2-dpf embryos were fixed in 4% paraformaldehyde in PBS overnight at 4°C. Fixed embryos were rinsed in PBS, bleached in 3% H2O2 for 30 min and stored in methanol at −20°C until use. For immunostaining, embryos were washed in PBS containing 0.1% Tween-20 and 0.5% Triton X-100 (PBSTX), and then permeabilization was achieved by incubation in deionized H2O for 60 min at RT followed by 100% acetone for 8 min at −20°C. Non-specific binding was blocked by incubation in 10% normal goat serum (NGS) and 3% BSA for 3 h at RT. After several washes with PBSTX, embryos were incubated in rabbit polyclonal anti 5-HT (ImmunoStar) diluted at 1:500 in 10% NGS/PBS containing 0.3% Triton-X 100 for 2 days at 4°C. After rinsing in PBSTX for 4 h, embryos were incubated in the goat anti-rabbit IgG Alexa Fluor 488 (Molecular Probes Invitrogen Detection Technologies) diluted at 1:100 in 10% NGS/PBS overnight at 4°C. After thorough washing in PBSTX, embryos were mounted in 0.5% agarose and observed under the fluorescence microscope (Leica M165 FC). Negative controls processed by omitting incubation with the primary antibody or by replacing the primary antibody with normal rabbit serum showed no positive signals. For measurement of 5-HT-positive area, focus was adjusted on the field with the largest positive area, and NIH ImageJ software was used to quantify manually outlined areas. Immunostaining was performed using five to eight embryos per group, and the experiments were done in triplicate.
RT-PCR
Total RNA was extracted from larvae at 4 and 7 dpf (25 larvae/group) using ISOGEN II (Nippon Gene) and treated with DNase free (Ambion). cDNA was synthesized from 1 µg total RNA using Reverse Transcription System (Promega). A total reaction volume of 25 µL containing 2× GoTaq Green Master Mix (Promega), 10 µM of each primer, and 1 µL cDNA was subjected to PCR using Program Temp Control System PC708 (Astec). β-Actin was used as an internal control. Amplification conditions and primer sequences are listed in Table 2. The amplified products were separated on a 2% agarose gel. Levels of mRNAs expression were analyzed by NIH ImageJ software and normalized by the expression level in the control group at each developmental time. Experiments were done in triplicate.
Heart Rate Measurement
Embryos at 2 dpf were individually placed in a well of a 12-well culture plate containing 500 µL of corresponding experimental medium and kept for 15 min to allow heartbeats to resume a steady rate. Heart beats were counted manually for 15 s under a stereo microscope (Leica 58APO). Ten embryos were used for each group. Experiments were repeated three times with eggs collected from different spawns.
Thigmotaxis Assay
Assay was performed according to the protocols described previously (49, 50). Briefly, zebrafish larvae at 6 dpf were transferred into a 6-well tissue culture plate with one fish per well containing 4 mL EM. The bottom of each well was divided into two portions designated as inner and outer zones. After habituation at 28°C for 2 h followed by acclimation under the video camera for 5 min, swimming activity was recorded for 5 min. For each group, 12 larvae were used. Data from 36 larvae from three different spawns were pooled and analyzed to express % of time a fish spent in the outer zone.
Statistical Analysis
Data are presented as mean ± SEM. Statistical differences between groups were evaluated by one-way ANOVA followed by Tukey’s or least significant difference post hoc test using IBM SPSS statistics version 19. Unpaired Student’s t-test was used for the dot blot analysis. Kruskal–Wallis test and Mann–Whitney U test were used for thigmotaxis assays, as the data did not meet the assumptions required for parametric testing. Significant differences were accepted when p < 0.05.
Results
Effect of E2 Exposure on Serotonergic Neuron
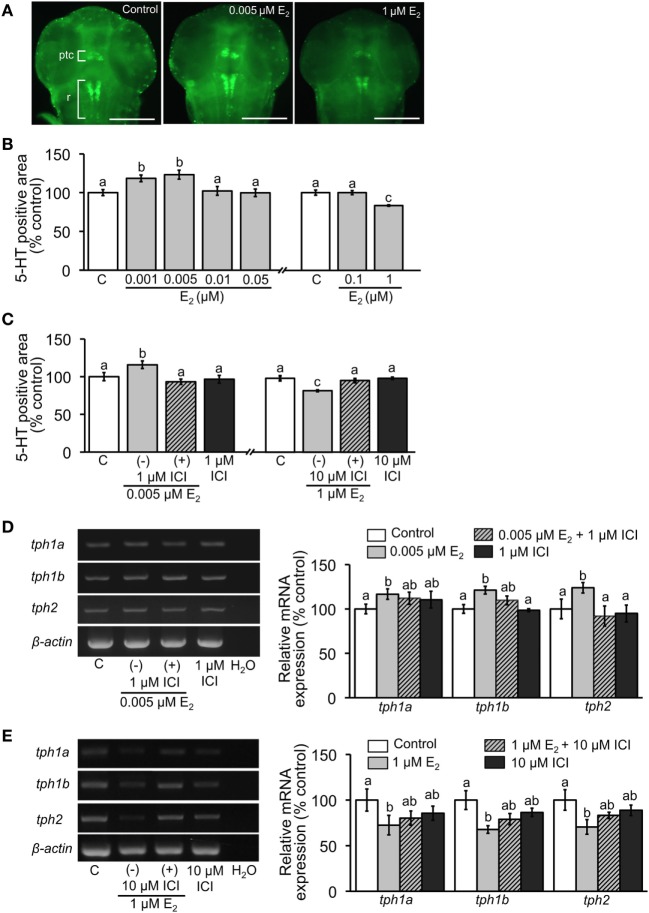
5-HT-positive neurons were detected in the embryos at 2 dpf by whole-mount fluorescent immunohistochemistry (Figure 1A). Positively stained neurons were located in pretectal and thalamic complex and raphe as reported previously (48, 51–54). 5-HT-positive areas were significantly increased when exposed to low doses E2 (0.001 and 0.005 µM) but decreased in high dose (1 µM) exposure (Figure 1B). Effects of both low and high doses E2 were significantly reversed by addition of 1 or 10 µM ICI, respectively (Figure 1C).
Figure 1.
Effect of E2 on 5-HT-positive area and relative expression of tph isoforms. (A) Representative images of ventral view of head region showing 5-HT-positive cells in pretectal and thalamic complex (ptc) and raphe (r) at 2 dpf. Scale bar: 200 µm. (B,C) Measurements of the area of 5-HT-positive neurons in the experiment of E2 exposure and co-incubation of E2 and ICI, respectively. (D,E) Semi-quantitative PCR for expression of tph isoforms in 4 dpf larvae in the experiments of exposure to low and high doses of E2 and co-incubation with ICI, respectively. Data are presented as a mean ± SEM. Different letters in each graph indicate significant differences (p < 0.05).
Relative expression levels of tph isoforms at 4 dpf were analyzed by semi-quantitative PCR. Significant increase in expression was detected in all tph isoforms when embryos were exposed to low-dose E2 (0.005 µM). Addition of 1 µM ICI completely reversed the decreased expression of tph2, while expressions of tph1a and tph1b were reversed partially (Figure 1D). Conversely, high-dose E2 exposure significantly decreased expression levels of all isoforms, which was partially reversed by addition of 10 μM ICI (Figure 1E).
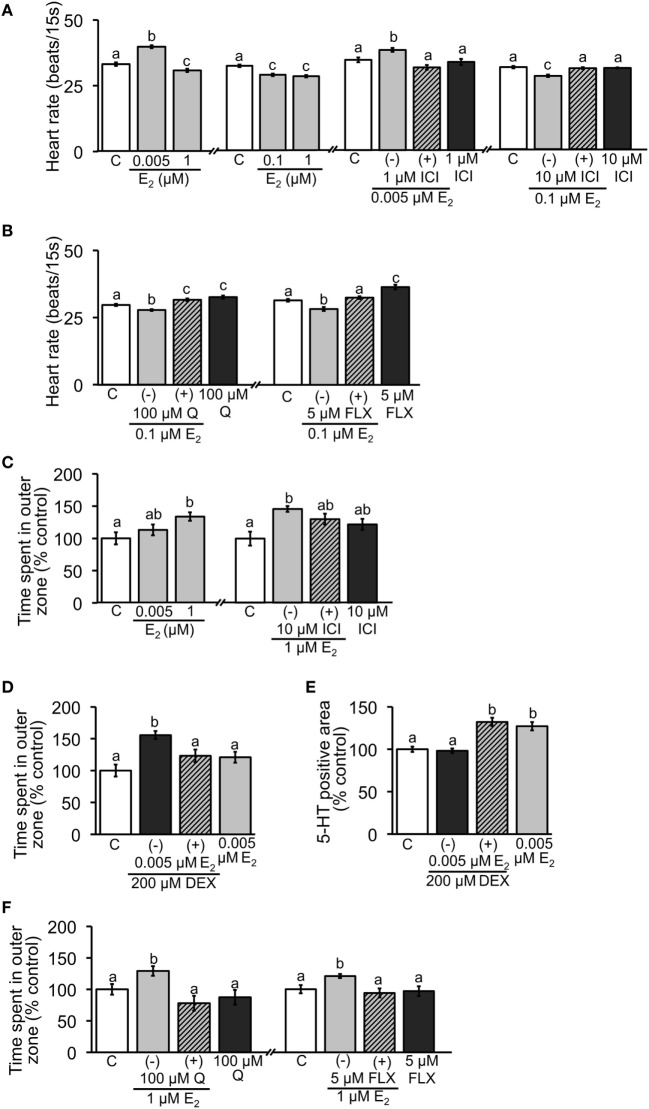
When the embryos were exposed to E2, the heart rate at 2 dpf was significantly increased in the 0.005 µM group, while significant decrease was found in the 0.1 and 1 µM groups (Figure 2A). Addition of 1 and 10 µM ICI significantly reversed the effects caused by low or high dose of E2, respectively (Figure 2A). To verify the role of serotonergic signaling in regulation of heart rate, effects of Q (5-HT agonist) and FLX (5-HT selective re-uptake inhibitor) were tested. Co-incubation with 0.1 µM E2 significantly reversed the decreased heart rate caused by E2. Heart rate was significantly increased when exposed to Q or FLX alone (Figure 2B).
Figure 2.
Effect of E2 on heart rate and thigmotaxis. Heart rate was measured in 2-dpf embryos; (A) exposure to low and high doses of E2 and co-incubation with ICI; (B) co-incubation of E2 and Q or FLX to examine involvement of serotonin signaling. Thigmotaxis assay was performed to evaluate anxiety level in 6-dpf larvae; (C) exposure to low and high doses of E2 and co-incubation with ICI; (D) anxiolytic effect of low dose of E2 when larvae were exposed to 200 µM DEX. (E) Measurements of 5-HT-positive neuron area in 2-dpf embryos exposed to DEX and low dose of E2. (F) Effects of co-incubation of E2 and Q or FLX on thigmotaxis to examine involvement of serotonin signaling. Data are presented as a mean ± SEM. Different letters in each graph indicate significant differences (p < 0.05).
Thigmotaxis assay was performed using 6-dpf larvae. Exposure to 1 µM E2 increased the time fish spent in outer zone, suggesting that anxiety was increased, but no significant difference was observed in 0.005 µM E2 group (Figure 2C). Addition of 10 µM ICI partially reversed the increase caused by 1 µM E2 (Figure 2C). To further examine the effect of low-dose E2, embryos were exposed to 200 µM DEX and subjected to the assay. DEX alone increased the time, but co-incubation with 0.005 µM E2 significantly reduced the increase (Figure 2D). Immunostaining for 5-HT showed that co-incubation of low-dose E2 and DEX increased the positive staining, although DEX alone had no effect (Figure 2E). To verify the role of serotonergic signaling in thigmotaxis assay, effects of Q and FLX were tested. Both Q and FLX significantly decreased the time fish spent in outer zone caused by 1 µM E2 exposure (Figure 2F).
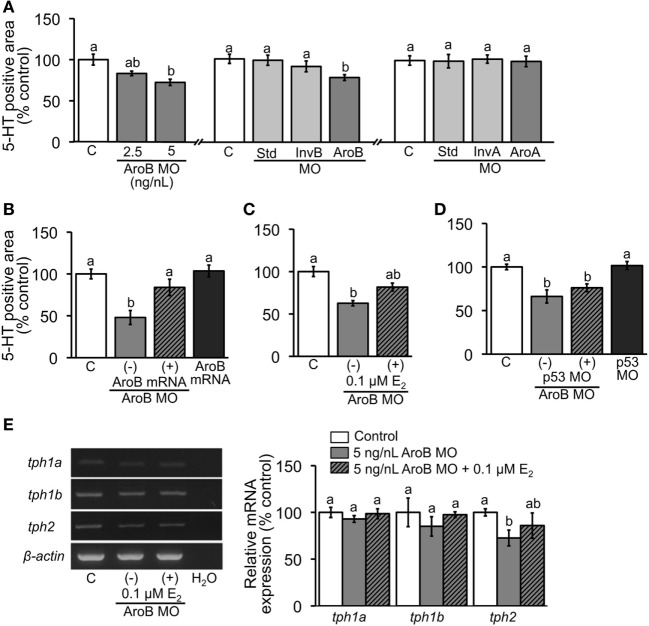
Validation of MO-Mediated Knockdown Using the Specific Antisera
Specificity of the antisera to AroB and AroA was examined by Western blot. The anti-AroB revealed a single band at the expected size of 50 kDa in brain extract from the fish exposed to E2 at 25 ng/L (47) (Figure 3A). The anti-AroA detected a single band at the expected size of 75 kDa in the ovarian extract (Figure 3A), which is in agreement with the previous study (55). In addition, immunohistochemistry of the ovary showed similar localization of AroA as previously described (56) (data not shown).
Figure 3.
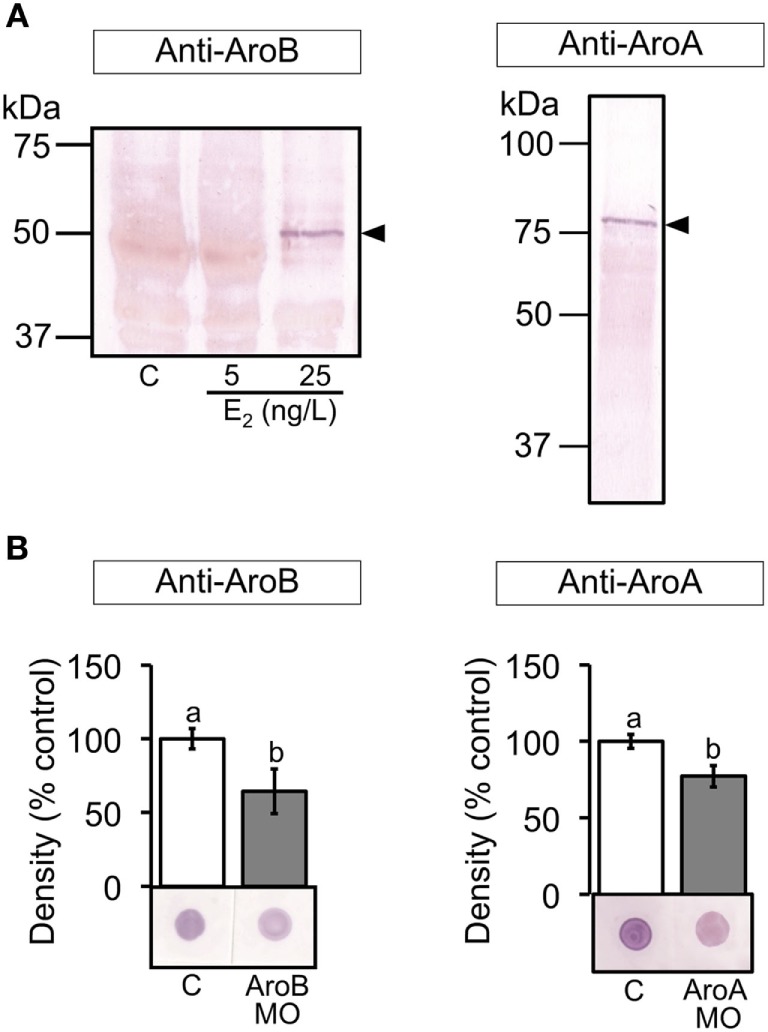
Validation of MO-mediated knockdown on translation. (A) Western blots of brain extracts from control and E2 exposed fish and ovarian extract from untreated fish were stained with the antiserum to AroB and AroA, respectively, showing a positive band at the expected size for each aromatase as indicated by arrowheads. (B) Dot blots of 6 and 2 dpf larval extracts using the antiserum to AroB and AroA, respectively, were analyzed. Representative blots are shown in each graph. Data are presented as a mean ± SEM. Different letters in each graph indicate significant differences (p < 0.05).
The dot blot analysis of the larval extracts showed that both AroB and AroA MO injections significantly decreased immunoreactivity compared to the uninjected control, indicating decreased translation of AroB and AroA, respectively (Figure 3B). Std MO, InvB, or InvA MO did not show any significant difference compared to the uninjected control (data not shown).
Effect of MO-Mediated Knockdown of AroB on Serotonergic Neuron
When AroB MO was injected, 5-HT-positive area was significantly decreased in the 5 ng/L group and partially decreased in the 2.5 ng/nL group compared to the uninjected control (Figure 4A). Injections of Std MO and InvB MO did not show any significant difference in 5-HT-positive areas compared to the uninjected control (Figure 4A). Moreover, the injection of AroA did not show any changes (Figure 4A). The decrease in 5-HT-positive area caused by AroB was completely rescued by co-injection of 30 pg/nL AroB mRNA (Figure 4B) and partially rescued by E2 exposure at 0.1 µM (Figure 4C). When p53 MO was co-injected with AroB MO to examine off-target effect, no significant difference in 5-HT-positive area was observed, suggesting that decrease in 5-HT-positive area caused by AroB MO is not due to apoptosis caused by p53 activation (Figure 4D).
Figure 4.
AroB MO-mediated effect on serotonergic neuron. 5-HT-positive neuron area was measured in 2-dpf embryos; (A) injection of AroB MO and control MOs (Std MO, InvB MO and AroA MO); (B,C) co-injection of AroB mRNA and exposure to E2, respectively, to rescue the effect of AroB MO; (D) co-injection of p53 MO to test off-target effect of AroB MO. (E) Semi-quantitative PCR measurement of expression of tph isoforms in 6-dpf larvae injected with AroB MO with and without exposure to E2. Data are presented as a mean ± SEM. Different letters in each graph indicate significant differences (p < 0.05).
The effect of AroB MO injection on relative expression of tph isoforms was evaluated by semi-quantitative PCR using 7-dpf larvae. While expression levels of tph1a and tph1b showed no significant changes, expression of tph2 isoform was significantly decreased and partially rescued by E2 exposure at 0.1 µM (Figure 4E).
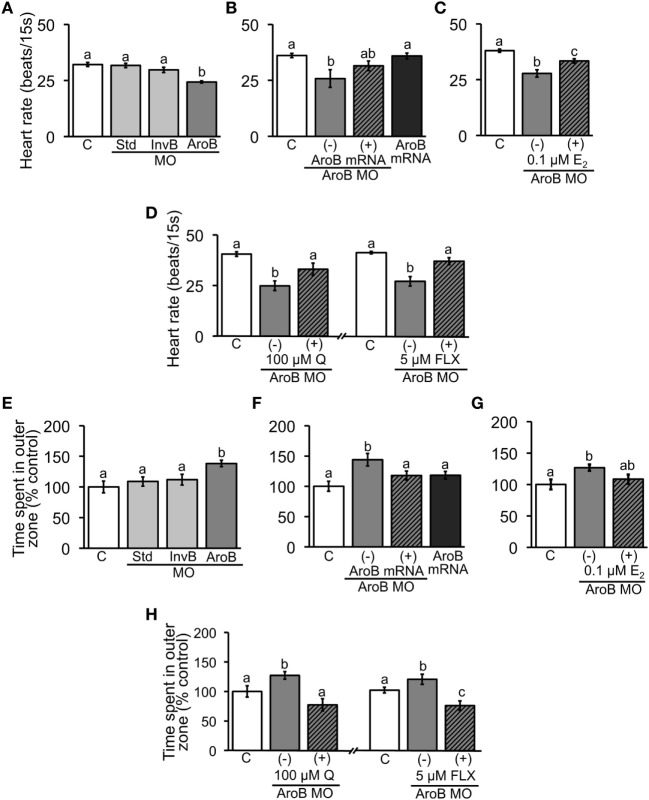
Heart rate of AroB MO injected embryos was significantly decreased compared to the uninjected or Std MO and InvB MO injected controls (Figure 5A). The decrease caused by AroB MO was rescued either by co-injection of AroB mRNA or by exposure to 0.1 µM E2 (Figures 5B,C). Exposure to 100 µM Q as well as to 5 µM FLX (Figure 5D) reversed the decrease to the control level.
Figure 5.
AroB MO-mediated effect on heart rate and thigmotaxis. Heart rate was measured in 2-dpf embryos; (A) injection of AroB MO and control MOs; (B,C) co-injection of AroB mRNA and exposure to E2, respectively, to rescue the effect of AroB MO; (D) injection of AroB MO with and without exposure to Q and FLX to examine involvement of serotonin signaling. Thigmotaxis assay was conducted using 6-dpf larvae; (E) injection of AroB MO and control MOs; (F,G) co-injection of AroB mRNA and exposure to E2, respectively, to rescue the effect of AroB MO; (H) injection of AroB MO with and without exposure to Q and FLX to examine involvement of serotonin signaling. Data are presented as a mean ± SEM. Different letters in each graph indicate significant differences (p < 0.05).
In thigmotaxis assay, AroB MO injection caused significant increase in time fish spent in outer zone compared to the uninjected, Std MO injected, and InvB MO injected controls (Figure 5E). This effect of AroB MO was rescued either by co-injection of AroB mRNA or by exposure to 0.1 µM E2 (Figures 5F,G). Exposure to 100 µM Q as well as to 5 µM FLX reversed the time fish spent in outer zone increased by AroB MO injection (Figure 5H).
Discussion
The aim of this study is to elucidate the role of estradiol and brain aromatase in modulation of serotonergic neurons in early development of zebrafish, as the early ontogeny of serotonergic system may be one of the important factors for neuronal growth and brain development. We demonstrated that exogenous administration of E2 biphasically affected parameters such as 5-HT-positive areas, relative expression of tph isoforms, heart rate and thigmotactic behavior with stimulation and suppression of serotonin system at the low dose and the high dose, respectively, through acting on ER. On the other hand, activities of serotonergic neurons were suppressed by AroB MO-mediated knockdown, suggesting that brain-formed E2 in early development stimulates serotonergic neurons, which is in accordance with the results of the low-dose E2. Recent study shows that MO-mediated brain aromatase knockdown results in a significant decrease in E2 concentration in 48 hpf embryos (57), which supports that our MO experiments reflect the reduction of estrogen production.
Non-monotonic dose responses of hormones and endocrine-disrupting chemicals have been widely documented (58). Estrogen among other hormones is known to exhibit biphasic dose-dependent effects in various physiological processes (59–66). However, only limited information is available in regards to serotonin system. There is one study in fish showing that low dose of E2 stimulated monoamine oxidase activity and decreased 5-HT content in hypothalamus in ovariectomized catfish, while the result was opposite for high dose (67). Our study demonstrates that biphasic dose-dependent effects of E2 on serotonergic neuron in fish, and shows that the effects or both low and high doses are mediated through ER, indicating physiological relevance. The effect of the low dose of E2, stimulating serotonergic neuron, is likely to reflect the role of endogenous E2 in embryos, as AroB MO-mediated effects demonstrate that brain-formed estrogen is necessary to maintain activity of serotonergic neuron in embryos. Mechanisms of biphasic responses are complex, but may be in part controlled by downregulation and desensitization of receptors (57, 68). Thus, effects of high doses of E2 on serotonergic neuron in this study may be due to downregulation/desensitization of ERs. Sequence analysis of the promoter region of zebrafish tph isoforms shows the presence of 1/2 ERE in the upstream of transcription start site in all isoforms, suggesting possible nuclear action of estrogen, though their functional analysis is yet to be reported. In human serotonergic cell line, binding of E2 and ERβ has been shown to directly interact with 1/2 ERE of tph2 promoter to elicit gene expression (39). In addition to the classical action of E2 on nuclear receptors membrane ERs plays an important role in brain (69, 70). Interaction between membrane ERs and the metabolic glutamate receptor in the brain provides a rapid and transient E2 action (71, 72). Membrane bound G-protein-coupled ER, GPER/GPR30, also known to be involved in modulating rapid non-genomic action of E2, plays a role in several brain areas (73). Estrogen action through GPR30 has been suggested in regulation of serotonergic neuron in mammals (74). Further studies are required to elucidate the mechanisms by which estrogen regulates serotonergic neuron in zebrafish.
Attenuation of serotonergic neuron by AroB MO-mediated knockdown clearly demonstrated that brain-formed estrogen is necessary to maintain the serotonin system to control heart rate and anxiety behavior in early development of zebrafish. Validity of AroB knockdown was supported by several lines of evidence. Immunoreactivity to the antiserum specific to AroB was decreased in AroB MO injected embryos. In addition to no significant effects found in the controls including standard MO, inverted AroB MO and AroA MO-injected embryos, AroB MO-mediated effects were rescued by co-injection of AroB mRNA and exposure to E2. Off-target effect of MO injection was also examined by knockdown of p53, showing that the decreased 5-HT-positive area caused by AroB MO is not through activation of p53. The decrease in 5-HT-positive area by AroB MO injection indicates that brain-formed estrogen stimulates 5-HT synthesis, which is in accordance with the stimulatory effect of low-dose E2. When the relative expressions of tph isoforms were examined in AroB MO injected embryos, only tph2 expression was significantly decreased by AroB MO, which is well supported by the previous studies showing tph2 but not tph1 is expressed in raphe 5-HT neurons (54, 75, 76). Expression of tph2 in 5-HT neurons in pretectal and hypothalamic complex starts to appear at 60 hpf (76). On the other hand, whereas in the exposure experiments, expressions of all isoforms were affected by E2; increased by low dose and decreased by high dose. The results support the previous studies reporting that tph2 expressed in brain is responsible for 5-HT synthesis in the zebrafish (27, 28, 54). Thus, we provide the evidence that brain-formed estrogen stimulates tph2 expression to maintain 5-HT content in the serotonin neuron. The effects of E2 exposure on tph isoforms indicate E2 also modulates serotonin biosynthesis in tissues outside the brain. 5-HT has been reported to be produced in various organs including intestine which is the major source of 5-HT in the body and TPH1 is responsible for its synthesis (54, 77). Investigation of estrogen regulation of serotonin production in intestine during development would be a future research interest.
The parameters of physiological functions of serotonin system, heart rate, and thigmotactic behavior were measure to verify the activity of serotonergic neuron. The results were in accordance to the changes in 5-HT levels in the neurons; the increased 5-HT levels are accompanied by the increased heart rate and decreased thigmotactic behavior, while the contrary was true for the decreased 5-HT levels. Serotonin is known to be involved in cardiovascular function, and the effect of central serotonergic neuron is mediated through autonomic nervous system in mammals (18). Our result of the low-dose (0.005 µM) E2 which increased heart rate corroborates the effect of MO-mediated AroB knockdown, indicating that nanomolar level of brain-formed estrogen, or even lower level in the tissue, stimulates serotonergic neuron to increase the heart rate. Exposure to quipazine (serotonin agonist), or fluoxetine (selective serotonin reuptake inhibitor, SSRI) completely reversed the decreased heart rate caused by the high dose (0.1 µM) E2, or AroB MO injection confirming that heart rate is under the control of serotonin signaling. Taken together with a recent study showing that GPER in the pituitary of zebrafish embryo regulates heart rate through thyroid hormone (78), estrogen in brain centrally regulates heart rate through various mechanisms. On the other hand, cardiac functions are directly regulated by estrogen (79) and aromatase has been detected in the heart tissues such as myocardium in mice (80–82). Therefore, it is possible that AroB MO injection may affect aromatase expression in the heart and locally produced estrogen modulates heart rate. In some teleost fish, both ovarian and brain aromatases are expressed in the heart (83–85), but in ricefield eel only brain aromatase is detected (86), while only ovarian aromatase is present in spotted scat (87). These difference may be due to technical difference as well as differences in species and developmental and physiological status. Our preliminary analysis indicated the expression of ovarian aromatase but not the brain form in adult zebrafish heart (data not shown), suggesting that our result of MO injection is likely to be mediated through knockdown of brain aromatase expressed in the brain not in the heart. However, expression of aromatase in the heart during development needs to be verified.
Thigmotaxis is an evolutionally conserved behavior associated with fear and has been shown to be affected by anxiolytic and anxiogenic compounds (88); thus, it has been used to measure anxiety levels in animals including fish (89–92). Our present study shows the high dose E2 (1 µM), which decreased the 5-HT level, significantly increased anxiety (increased time spent in outer zone), and this increase was abolished by addition of 5-HT agonist (Q) or SSRI (FLX), indicating the effect of high dose E2 is mediated through serotonin signaling. Similarly, increased anxiety by AroB MO was also abolished by Q or FLX, which supports our hypothesis that brain-formed estrogen modulates serotonergic neuron. Despite our expectation, the low-dose E2 (0.005 µM), which increased the 5-HT level, did not cause reduction of anxiety. Therefore, we further examined to see if the low-dose E2 exerts anxiolytic effect in the larvae exposed to DEX to induce stress, and indeed, low-dose E2 decreased the anxiety level. Thus, our data demonstrate a negative correlation between anxiety behavior and 5-HT level, which is in accordance with previous studies. In mammals, depletion of 5-HT level in rat brain induces anxiety (93) and acute reduction of tryptophan increases the anxiety level in patients of a social anxiety disorder (94). The role of 5-HT in anxiety is also reported in zebrafish (20, 95). Buspirone, partial agonist for 5-HT1A receptor, exerts anxiolytic-like effect in zebrafish (96). The phenotype of zebrafish leopard strain, which is characterized by increased anxiety-like behavior, is rescued by acute treatment with FLX (97). Taken together, we provide the evidence that brain-formed E2 has an important role in modulating anxiety through serotonergic transmission.
In contrast to mammalian brain, where aromatase is expressed in both neuron and glia (98, 99), it is well documented that brain aromatase in fish is exclusively expressed in RGCs along the ventricles of forebrain, midbrain, and hindbrain serving as neural progenitors (10, 11, 47). While most RGCs are transformed into astrocytes by the time of adulthood in mammalian brain (100), presence of RGCs persists throughout the lifespan of zebrafish, which is considered to be one of the contributing factors for high capacity of neuronal proliferation (101). On the other hand, serotonin is known to play a role in neurogenesis (102). In adult zebrafish, it has been reported that projection of 5-HT neurons in raphe to ventricular surface of the brain, where highly proliferative cells are found. In addition, expression of 5-HT receptors are localized in ventricular surface in larval and adult zebrafish (27, 103). Thus, it may be possible that RGCs in ventricular surface are innervated by 5-HT neurons in raphe and modulated for neurogenesis. Interestingly, it has been reported that AroB-positive RGCs in PVO area in adult zebrafish has an ability to differentiate into serotonergic neuron (104). Taken together with our present study providing the evidence that brain-formed estrogen is necessary to maintain the levels of 5-HT in neurons in raphe, we can hypothesize that differentiation of AroB-expressing RGCs in serotonin neurons is regulated by serotonin neuron in raphe, whose activity is modulated by estrogen produced by AroB. It has been shown that placenta aromatase activity and expression are stimulated by serotonergic 5-HT2A receptor signaling (105). In goldfish, AroB expression in RGCs in vitro is upregulated by dopamine with modulation by E2 (106). Nonetheless, estrogen biosynthesis and homeostasis in CNS are regulated and fine-tuned by multiple factors like neurotransmitters and hormones, so that diverse functions of estrogen can be coordinated.
In conclusion, this study demonstrates that estradiol exhibits a biphasic effect on serotonergic neuron, and that brain aromatase, thus brain-formed estrogen plays a significant role in modulating serotonin levels to sustain appropriate development and functions of serotonergic neurons which regulate heart rate and anxiety behavior in zebrafish embryos and larvae. Considering the role of serotonergic neurons in neural development and neurogenesis, it is possible to postulate that one of the mechanisms of brain aromatase and brain-formed estrogen to regulate neurogenesis in teleost brain may be through modulation of serotonergic system, which awaits future investigation.
Ethics Statement
All experimental procedures and maintenance of fish were conducted in accordance with the Guide for Care and Use of Laboratory Animals published by the US National Institutes of Health.
Author Contributions
ZU and MK designed the experiments. ZU performed the experiments and analyzed the data. ZU and MK wrote the paper.
Conflict of Interest Statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Acknowledgments
ZU was supported by MEXT (Ministry of Education, Culture, Sports, Science and Technology, Japan) scholarship. We would like to thank Prof. Akiyoshi Takahashi and Assoc. Prof. Kanta Mizusawa for helping ZU to generate AroB mRNA, and Prof. Kazufumi Takamune for his support with western blot experiments.
References
- 1.Simpson ER, Davis SR. Minireview: aromatase and the regulation of estrogen biosynthesis-some new perspectives. Endocrinology (2001) 142(11):4589–94. 10.1210/endo.142.11.8547 [DOI] [PubMed] [Google Scholar]
- 2.Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during ageing: from periphery to brain. Trends Mol Med (2013) 19(3):197–209. 10.1016/j.molmed.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kishida M, Callard GV. Distinct cytochrome P450 aromatase isoforms in zebrafish (Danio rerio) brain and ovary are differentially programmed and estrogen regulated during early development. Endocrinology (2001) 142(2):740–50. 10.1210/endo.142.2.7928 [DOI] [PubMed] [Google Scholar]
- 4.Callard GV, Greytak SR, Novillo A, Cotter CA, Mayer RK. Brain aromatase in fish: perspective and comparative approaches. In: Balthazart J, Ball G, editors. Brain Aromatase, Estrogen, and Behavior. New York: Oxford University Press; (2013). p. 13–42. [Google Scholar]
- 5.Pasmanik M, Callard GV. Changes in brain aromatase and 5 alpha-reductase activities correlate significantly with seasonal reproductive cycles in goldfish (Carassius auratus). Endocrinology (1988) 122(4):1349–56. 10.1210/endo-122-4-1349 [DOI] [PubMed] [Google Scholar]
- 6.Zupanc GKH. Adult neurogenesis and neuronal regeneration in the brain of teleost fish. J Physiol Paris (2008) 102(4–6):357–73. 10.1016/j.jphysparis.2008.10.007 [DOI] [PubMed] [Google Scholar]
- 7.Adolf B, Chapouton P, Lam CS, Topp S, Tannhäuser B, Strähle U, et al. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev Biol (2006) 295(1):278–93. 10.1016/j.ydbio.2006.03.023 [DOI] [PubMed] [Google Scholar]
- 8.Grandel H, Kaslin J, Ganz J, Wenzel I, Brand M. Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev Biol (2006) 295(1):263–77. 10.1016/j.ydbio.2006.03.040 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt R, Strähle U, Scholpp S. Neurogenesis in zebrafish – from embryo to adult. Neural Dev (2013) 8:3. 10.1186/1749-8104-8-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forlano PM, Deitcher DL, Myers DA, Bass AH. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: aromatase enzyme and mRNA expression identify glia as source. J Neurosci (2001) 21(22):8943–55. 10.1523/JNEUROSCI.21-22-08943.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong SK, Mouriec K, Kuo MW, Pellegrini E, Gueguen MM, Brion F, et al. A cyp19a1b-gfp (aromatase B) transgenic zebrafish line that expresses GFP in radial glial cells. Genesis (2009) 47:67–73. 10.1002/dvg.20459 [DOI] [PubMed] [Google Scholar]
- 12.Radakovits R, Barros CS, Belvindrah R, Patton B, Müller U. Regulation of radial glial survival by signals from the meninges. J Neurosci (2009) 29(24):7694–705. 10.1523/JNEUROSCI.5537-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawyer SJ, Gerstner KA, Callard GV. Real-time PCR analysis of cytochrome P450 aromatase expression in zebrafish: gene specific tissue distribution, sex differences, developmental programming, and estrogen regulation. Gen Comp Endocrinol (2006) 147(2):108–17. 10.1016/j.ygcen.2005.12.010 [DOI] [PubMed] [Google Scholar]
- 14.Diotel N, Vaillant C, Gabbero C, Mironov S, Fostier A, Gueguen MM, et al. Effects of estradiol in adult neurogenesis and brain repair in zebrafish. Horm Behav (2013) 63(2):193–207. 10.1016/j.yhbeh.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 15.Graeff FG, Guimarães FS, De Andrade TGCS, Deakin JFW. Role of 5-HT in stress, anxiety and depression. Pharmacol Biochem Behav (1996) 54(1):129–41. 10.1016/0091-3057(95)02135-3 [DOI] [PubMed] [Google Scholar]
- 16.Michelsen KA, Schmitz C, Steinbusch HWM. The dorsal raphe nucleus-from silver stainings to a role in depression. Brain Res Rev (2007) 55(2):329–42. 10.1016/j.brainresrev.2007.01.002 [DOI] [PubMed] [Google Scholar]
- 17.Bravo JA, Dinan TG, Cryan JF. Early-life stress induces persistent alterations in 5-HT1A receptor and serotonin transporter mRNA expression in the adult rat brain. Front Mol Neurosci (2014) 7(24):1–9. 10.3389/fnmol.2014.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Côté F, Fligny C, Fromes Y, Mallet J, Vodjdani G. Recent advances in understanding serotonin regulation of cardiovascular function. Trends Mol Med (2004) 10(5):232–8. 10.1016/j.molmed.2004.03.007 [DOI] [PubMed] [Google Scholar]
- 19.Nakamura K. The role of the dorsal raphé nucleus in reward-seeking behavior. Front Integr Neurosci (2013) 7(60):1–18. 10.3389/fnint.2013.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herculano AM, Maximino C. Serotonergic modulation of zebrafish behavior: towards a paradox. Prog Neuropsychopharmacol Biol Psychiatry (2014) 55:50–66. 10.1016/j.pnpbp.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 21.Berg DA, Belnoue L, Song H, Simon A. Neurotransmitter mediated control of neurogenesis in the adult vertebrate brain. Development (2013) 140(2):2548–61. 10.1242/dev.088005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charnay Y, Léger L. Brain serotonergic circuitries. Dialogues Clin Neurosci (2010) 12(4):471–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesch KP, Waider J. Serotonin in the modulation of neural plasticity and networks: implications for neurodevelopmental disorders. Neuron (2012) 76(1):175–91. 10.1016/j.neuron.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 24.Herlenius E, Lagercrantz H. Neurotransmitters and neuromodulators during early human development. Early Hum Dev (2001) 65(1):21–37. 10.1016/S0378-3782(01)00189-X [DOI] [PubMed] [Google Scholar]
- 25.Sodhi MS, Sanders-Bush E. Serotonin and brain development. Int Rev Neurobiol (2004) 59:111–74. 10.1016/S0074-7742(04)59006-2 [DOI] [PubMed] [Google Scholar]
- 26.Sundström E, Kolare S, Souverbie F, Samuelsson EB, Pschera H, Lunell NO, et al. Neurochemical differentiation of human bulbospinal monoaminergic neurons during the first trimester. Brain Res Dev Brain Res (1993) 75(1):1–12. 10.1016/0165-3806(93)90059-J [DOI] [PubMed] [Google Scholar]
- 27.Lillesaar C. The serotonergic system in fish. J Chem Neuroanat (2011) 41(4):294–308. 10.1016/j.jchemneu.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 28.Gaspar P, Lillesaar C. Probing the diversity of serotonin neurons. Philos Trans R Soc Lond B Biol Sci (2012) 367(1601):2382–94. 10.1098/rstb.2011.0378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barreiro-Iglesias A, Mysiak KS, Scott AL, Reimer MM, Yang Y, Becker CG, et al. Serotonin promotes development and regeneration of spinal motor neurons in zebrafish. Cell Rep (2015) 13(5):924–32. 10.1016/j.celrep.2015.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McEwen BS, Elves SE. Estrogen actions in the central nervous system. Endocr Rev (1999) 20(3):279–307. 10.1210/edrv.20.3.0365 [DOI] [PubMed] [Google Scholar]
- 31.Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol (2002) 23(1):41–100. 10.1006/frne.2001.0225 [DOI] [PubMed] [Google Scholar]
- 32.Pecins-Thompson M, Brown NA, Kohama SG, Bethea CL. Ovarian steroid regulation of tryptophan hydroxylase mRNA expression in rhesus macaque. J Neurosci (1996) 16(21):7021–9. 10.1523/JNEUROSCI.16-21-07021.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pecins-Thompson M, Brown NA, Bethea CL. Regulation of serotonin re-uptake transporter mRNA expression by ovarian steroids in rhesus macaques. Brain Res Mol Brain Res (1998) 53(1–2):120–9. 10.1016/S0169-328X(97)00286-6 [DOI] [PubMed] [Google Scholar]
- 34.Pecins-Thompson M, Bethea CL. Ovarian steroid regulation of serotonin-1A autoreceptor messenger RNA expression in the dorsal raphe of rhesus macaques. Neuroscience (1999) 89(1):267–77. 10.1016/S0306-4522(98)00326-1 [DOI] [PubMed] [Google Scholar]
- 35.Gundlah C, Lu NZ, Mirkes SJ, Bethea CL. Estrogen receptor beta (ERβ) mRNA and protein in serotonin neurons of macaques. Mol Brain Res (2001) 91:14–22. 10.1016/S0169-328X(01)00108-5 [DOI] [PubMed] [Google Scholar]
- 36.Sheng Z, Kawano J, Yanai A, Fujinaga R, Tanaka M, Watanabe Y, et al. Expression of estrogen receptors (α, β) and androgen receptor in serotonin neurons of the rat and mouse dorsal raphe nuclei; sex and species differences. Neurosci Res (2004) 49:185–96. 10.1016/j.neures.2004.02.011 [DOI] [PubMed] [Google Scholar]
- 37.Bethea CL, Phu K, Belikova Y, Bethea SC. Localization and regulation of reproductive steroid receptors in the raphe serotonin system of male macaques. J Chem Neuroanat (2015) 6(6–67):19–27. 10.1016/j.jchemneu.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donner N, Handa RJ. Estrogen receptor beta regulates the expression of tryptophanhydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience (2009) 163(2):705–18. 10.1016/j.neuroscience.2009.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiroi R, Handa RJ. Estrogen receptor-β regulates human tryptophan hydroxylase-2 through an estrogen response element in the 5’ untranslated region. J Neurochem (2013) 127(4):487–95. 10.1111/jnc.12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai CL, Wang LH, Chang CF, Kao CC. Effects of gonadal steroids on brain serotonergic and aromatase activity during the critical period of sexual differentiation in tilapia, Oreochromis mossambicus. J Neuroendocrinol (2000) 12(9):894–8. 10.1046/j.1365-2826.2000.00536.x [DOI] [PubMed] [Google Scholar]
- 41.Thilagam H, Gopalakrishnan S, Bo J, Wang KJ. Comparative study of 17β-estradiol on endocrine disruption and biotransformation in fingerlings and juveniles of Japanese sea bass Lateolabrax japonicas. Mar Pollut Bull (2014) 85:332–7. 10.1016/j.marpolbul.2014.05.024 [DOI] [PubMed] [Google Scholar]
- 42.Diotel N, Do Rego JL, Anglade I, Vaillant C, Pellegrini E, Vaudry H, et al. The brain of teleost fish, a source, and a target of sexual steroids. Front Neurosci (2011) 5:137. 10.3389/fnins.2011.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coumailleau P, Pellegrini E, Adrio F, Diotel N, Nicolau JC, Nasri A, et al. Aromatase, estrogen receptors and brain development in fish and amphibians. Biochim Biophys Acta (2015) 1849(2):152–62. 10.1016/j.bbagrm.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 44.Langheinrich U, Hennen E, Stott G, Vacun G. Zebrafish as a model organism for the identification and characterization of drugs and genes affecting p53 signaling. Curr Biol (2002) 12(23):2023–8. 10.1016/S0960-9822(02)01319-2 [DOI] [PubMed] [Google Scholar]
- 45.Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, et al. p53 activation by knockdown technologies. PLoS Genet (2007) 3(5):e78. 10.1371/journal.pgen.0030078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bashammakh S. The Developmental Role of the Serotonin System [Dissertation Thesis]. Berlin: Freie Universität Berlin; (2007). Available from: http://edocs.fu-berlin.de/diss/receive/FUDISS_thesis_000000003162 [Google Scholar]
- 47.Menuet A, Pellegrini E, Brion F, Gueguen MM, Anglade I, Pakdel F, et al. Expression and estrogen-dependent regulation of the zebrafish brain aromatase gene. J Comp Neurol (2005) 485(4):304–20. 10.1002/cne.20497 [DOI] [PubMed] [Google Scholar]
- 48.Aihart MJ, Lee DH, Wilson TD, Miller BE, Miller MN, Skalko RG, et al. Adverse effects of serotonin depletion in developing zebrafish. Neurotoxicol Teratol (2012) 34(1):152–60. 10.1016/j.ntt.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 49.Jannat M, Sugiyono, Kishida M. Nitrite (NO2-) perturbs the activitu of serotonergic neuron during early development of zebrafish. Int J Eng Res Sci Tech (2014) 3(4):1–11. [Google Scholar]
- 50.Norton WHJ. Measuring larval zebrafish behavior: locomotion, thigmotaxis, and startle. In: Kalueff AV, Stewart AM, editors. Zebrafish Protocols for Neurobehavioral Research. Totowa, NJ: Humana Press; (2012). p. 3–19. [Google Scholar]
- 51.Brustein E, Chong M, Holmqvist B, Drapeau P. Serotonin patterns locomotor network activity in the developing zebrafish by modulating quiescent periods. J Neurobiol (2003) 57(3):303–22. 10.1002/neu.10292 [DOI] [PubMed] [Google Scholar]
- 52.Holzschuh J, Gimeno AB, Ettl AK, Dürr K, Knapik EW, Driever W. Noradrenergic neurons in the zebrafish hindbrain are induced by retinoic acid and require tfap2a for expression of the neurotransmitter phenotype. Development (2003) 130(23):5741–54. 10.1242/dev.00816 [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Takai R, Yoshioka H, Shirabe K. Characterization and expression of serotonin transporter genes in zebrafish. Tohoku J Exp Med (2006) 208(3):267–74. 10.1620/tjem.208.267 [DOI] [PubMed] [Google Scholar]
- 54.Bashammakh S, Wurtele M, Kotnik K, Seyfried SA, Bader M. Serotonin is required for pharyngeal arch morphogenesis in zebrafish. ScienceOpen Res (2015):1–9. 10.14293/S2199-1006.1.SOR-LIFE.AWPDLZ.v1 [DOI] [Google Scholar]
- 55.Liu W, Chen C, Chen L, Wang L, Li J, Chen Y, et al. Sex-dependent effects of microcystin-LR on hypothalamic-pituitary-gonad axis and gametogenesis of adult zebrafish. Sci Rep (2016) 6:22819. 10.1038/srep22819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caulier M, Brion F, Chadili E, Turies C, Piccini B, Porcher JM, et al. Localization of steroidogenic enzymes and Foxl2a in the gonads of mature zebrafish (Danio rerio). Comp Biochem Physiol A Mol Integr Physiol (2015) 188:96–106. 10.1016/j.cbpa.2015.06.016 [DOI] [PubMed] [Google Scholar]
- 57.Alharthy KM, Albaqami FF, Thornton C, Corrales J, Willett KL. Mechanistic evaluation of benzo[a]pyrene’s developmental toxicities mediated by reduced cyp19a1b activity. Toxicol Sci (2017) 155(1):135–47. 10.1093/toxsci/kfw182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vandenberg LN, Colborn T, Heyes TB, Heindel JJ, Jacobs DR, Lee D, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev (2012) 33(3):378–455. 10.1210/er.2011-1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Voss AK, Fortune JE. Estradiol-17-β has a biphasic effect on oxytocin secretion by bovine granulosa cells. Biol Reprod (1993) 48:1404–9. 10.1095/biolreprod48.6.1404 [DOI] [PubMed] [Google Scholar]
- 60.Sobel MI, Winkel CA, Macy LB, Liao P, Bjornsson TD. The regulation of plasminogen activators and plasminogen activator inhibitor type 1 in endothelial cells by sex hormones. Am J Obstet Gynecol (1995) 173:801–8. 10.1016/0002-9378(95)90344-5 [DOI] [PubMed] [Google Scholar]
- 61.Banerjee SK, Campbell DR, Weston AP, Banerjee DK. Biphasic estrogen response on bovine adrenal medulla capillary endothelial cell adhesion, proliferation and tube formation. Mol Cell Biochem (1997) 177:97–105. 10.1023/A:100688802 [DOI] [PubMed] [Google Scholar]
- 62.Weiss-Messer E, Ber R, Barkey RJ. Prolactin and MA-10 Leydig cell steroidogenesis: biphasic effects of prolactin and signal transduction. Endocrinology (1996) 137:5509–18. 10.1210/endo.137.12.8940378 [DOI] [PubMed] [Google Scholar]
- 63.Yokoyama K, Hayashi M, Mogi C, Sasakawa Y, Watanabe G, Taya K, et al. Dose-dependent effects of a glucocorticoid on protein production. Endocr J (2008) 55(2):405–14. 10.1507/endocrj.K07E-063 [DOI] [PubMed] [Google Scholar]
- 64.Mazziotti G, Giustina A. Glucocorticoids and the regulation of growth hormone secretion. Nat Rev Endocrinol (2013) 9:265–76. 10.1038/nrendo.2013.5 [DOI] [PubMed] [Google Scholar]
- 65.Zhang L, Yulong M, Qin P, Deng Y, Zhang Z, Hou Y, et al. The effects of various estrogen doses on the proliferation and differentiation of cultured neural stem cells. J Cell Sci Ther (2016) 7:247. 10.4172/2157-7013.1000247 [DOI] [Google Scholar]
- 66.Houshmand F, Faghihi M, Imani A, Kheiri S. Effect of different doses of oxytocin on cardiac electrophysiology and arrhythmias induced by ischemia. J Adv Pharm Technol Res (2017) 8(4):131–7. 10.4103/japtr.JAPTR_178_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Senthilkumaran B, Joy KP. Effects of ovariectomy and oestradiol replacement on hypothalamic serotonergic and monoamine oxidase activity in the catfish, Heteropneustes fossilis: a study correlating plasma oestradiol and gonadotrophin levels. J Endocrinol (1994) 142(2):193–203. 10.1677/joe.0.1420193 [DOI] [PubMed] [Google Scholar]
- 68.Oliveira CA, Mehecha GAB, Carnes K, Prins GS, Saunders PTK, França L, et al. Differential hormonal regulation of estrogen receptors ERα and ERβ and androgen receptor expression in rat efferent ductules. Reproduction (2004) 128(1):73–86. 10.1530/rep.1.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCarthy MM. Estradiol and the developing brain. Physiol Rev (2008) 88(1):91–134. 10.1152/physrev.00010.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Micevych PE, Mermelstein PG, Sinchak K. Estradiol membrane-initiated signaling in the brain mediates reproduction. Trends Neurosci (2017) 40(11):654–66. 10.1016/j.tins.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boulware MI, Mermelstein PG. Membrane estrogen receptors activate metabotropic glutamate receptors to influence nervous system physiology. Steroids (2009) 74(7):608–13. 10.1016/j.steroids.2008.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cover KK, Maeng LY, Lebrón-Milad K, Milad MR. Mechanisms of estradiol in fear circuitry: implications for sex differences in psychopathology. Transl Psychiatry (2014) 5(4):e422. 10.1038/tp.2014.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Micevych PE, Kelly MJ. Membrane estrogen receptor regulation of hypothalamic function. Neuroendocrinology (2012) 96(2):103–10. 10.1159/000338400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu H, Qin S, Carassco GA, Dai Y, Filardo EJ, Prossnitz ER, et al. Extra-nuclear estrogen receptor GPR30 regulates serotonin function in rat hypothalamus. Neuroscience (2009) 158(4):1599–607. 10.1016/j.neuroscience.2008.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Teraoka H, Russell C, Regan J, Chandrasekhar A, Concha ML, Yokoyama R, et al. Hedgehog and Fgf signaling pathways regulate the development of tphR-expressing serotonergic raphe neurons in zebrafish embryos. J Neurobiol (2004) 60(3):275–88. 10.1002/neu.20023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lillesaar C, Tannhäuser B, Stigloher C, Kremmer E, Bally-Cuif L. The serotonergic phenotype is acquired by converging genetic mechanisms within the zebrafish central nervous system. Dev Dyn (2007) 236(4):1072–84. 10.1002/dvdy.21095 [DOI] [PubMed] [Google Scholar]
- 77.Côté F, Thévenot E, Fligny C, Fromes Y, Darmon M, Ripoche MA, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci U S A (2003) 100(23):13525–30. 10.1073/pnas.2233056100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Romano SN, Edwards HE, Sounder JP, Ryan KJ, Cui X, Gorelick DA. G protein-coupled estrogen receptor regulates embryonic heart rate in zebrafish. PLoS Genet (2017) 13(10):e1007069. 10.1371/journal.pgen.1007069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Babiker FA, De Windt LJ, van Eickels M, Grohe C, Meyer R, Doevendans PA. Estrogenic hormone action in the heart: regulatory network and function. Cardiovasc Res (2002) 53(3):709–19. 10.1016/S0008-6363(01)00526-0 [DOI] [PubMed] [Google Scholar]
- 80.Bell JR, Mellor KM, Wollermann AC, Ip WT, Reichelt ME, Meachem SJ, et al. Aromatase deficiency confers paradoxical postischemic cardioprotection. Endocrinology (2011) 152(12):4937–47. 10.1210/en.2011-1212 [DOI] [PubMed] [Google Scholar]
- 81.Jazbutyte V, Stumpner J, Redel A, Lorenzen JM, Roewer N, Thum T, et al. Aromatase inhibition attenuates desflurane-induced preconditioning against acute myocardial infarction in male mouse heart in vivo. PLoS One (2012) 7(8):e42032. 10.1371/journal.pone.0042032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Connernet JJ, Spratt DI. Aromatase blockade is associated with increased mortality in acute illness in male mice. J Endocr Soc (2017) 1(9):1113–9. 10.1210/js.2017-00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chang X, Kobayashi T, Senthilkumaran B, Kobayashi-Kajura H, Sudhakumari CC, Nagahama Y. Two types of aromatase with different encoding genes, tissue distribution and developmental expression in Nile tilapia (Oreochromis niloticus). Gen Comp Endocrinol (2005) 141(2):101–15. 10.1016/j.ygcen.2004.11.020 [DOI] [PubMed] [Google Scholar]
- 84.Choi JY, Park JG, Jeong HB, Lee YD, Takemura A, Kim SJ. Molecular cloning of cytochrome P450 aromatases in the protogynous wrasse, Halichoeres tenuispinis. Comp Biochem Physiol B Biochem Mol Biol (2005) 141(1):49–59. 10.1016/j.cbpc.2005.01.009 [DOI] [PubMed] [Google Scholar]
- 85.Matsuoka MP, van Nes S, Andersen Ø, Benfey TJ, Reith M. Real-time PCR analysis of ovary- and brain-type aromatase gene expression during Atlantic halibut (Hippoglossus hippoglossus) development. Comp Biochem Physiol B Biochem Mol Biol (2006) 144(1):128–35. 10.1016/j.cbpb.2006.02.008 [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y, Zhang W, Yang H, Zhou W, Hu C, Zhang L. Two cytochrome P450 aromatase genes in the hermaphrodite ricefield eel Monopterus albus: mRNA expression during ovarian development and sex change. J Endocrinol (2008) 199(2):317–31. 10.1677/JOE-08-0303 [DOI] [PubMed] [Google Scholar]
- 87.Liu H, Mu X, Gui L, Su M, Li H, Zhang G, et al. Characterization and gonadal expression of FOXL2 relative to Cyp19a genes in spotted scat Scatophagus argus. Gene (2015) 561(1):6–14. 10.1016/j.gene.2014.12.060 [DOI] [PubMed] [Google Scholar]
- 88.Walz N, Mühlberger A, Pauli P. A human open field test reveals thigmotaxis related to agoraphobic fear. Biol Psychiatry (2016) 80(5):390–7. 10.1016/j.biopsych.2015.12.016 [DOI] [PubMed] [Google Scholar]
- 89.Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav (1988) 31:959–62. 10.1016/0091-3057(88)90413-3 [DOI] [PubMed] [Google Scholar]
- 90.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol (2003) 463:3–33. 10.1016/S0014-2999(03)01272-X [DOI] [PubMed] [Google Scholar]
- 91.Schönrr SJ, Steenbergen PJ, Richardson MK, Champagne DL. Measuring thigmotaxis in larval zebrafish. Behav Brain Res (2012) 228(2):367–74. 10.1016/j.bbr.2011.12.016 [DOI] [PubMed] [Google Scholar]
- 92.Champagne DL, Hoefnagels CC, de Kloet RE, Richardson MK. Translating rodent behavioral repertoire to zebrafish (Danio rerio): relevance for stress research. Behav Brain Res (2010) 214(2):332–42. 10.1016/j.bbr.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 93.Gurtman CG, Morley KC, Li KM, Hunt GE, McGregor IS. Increased anxiety in rats after 3,4-methylenedioxymethamphetamine: association with serotonin depletion. Eur J Pharmacol (2002) 446(1–3):89–96. 10.1016/S0014-2999(02)01820-4 [DOI] [PubMed] [Google Scholar]
- 94.Argyropoulos SV, Hood SD, Adrover M, Bell CJ, Rich AS, Nash JR, et al. Tryptophan depletion reverses the therapeutic effect of selective serotonin reuptake inhibitors in social anxiety disorder. Biol Psychiatry (2004) 56(7):503–9. 10.1016/j.biopsych.2004.07.006 [DOI] [PubMed] [Google Scholar]
- 95.Jia M, Pittman J. Deficits in striatal dopamine and hippocampal serotonin following induction of anxiety/depressive-like behaviors by bisphenol A. Arch Neurosci (2014) 2(1):e18555. 10.5812/archneurosci.18555 [DOI] [Google Scholar]
- 96.Gebauer DL, Pagnussat N, Piato AL, Schaefer IC, Bonan CD, Lara DR. Effects of anxiolytics in zebrafish: similarities and differences between benzodiazepines, buspirone and ethanol. Pharmacol Biochem Behav (2011) 99(3):480–6. 10.1016/j.pbb.2011.04.021 [DOI] [PubMed] [Google Scholar]
- 97.Maximino C, Puty B, Matos Oliveira KR, Herculano AM. Behavioral and neurochemical changes in the zebrafish leopard strain. Genes Brain Behav (2013) 12(5):576–82. 10.1111/gbb.12047 [DOI] [PubMed] [Google Scholar]
- 98.Martínez-Cerdeño V, Noctor SC, Kriegstein AR. Estradiol stimulates progenitor cell division in the ventricular and subventricular zones of the embryonic neocortex. Eur J Neurosci (2006) 24(12):3475–88. 10.1111/j.1460-9568.2006.05239.x [DOI] [PubMed] [Google Scholar]
- 99.Yague JG, Muñoz A, de Monasterio-Schrader P, Defelipe J, Garcia-Segura LM, Azcoitia I. Aromatase expression in the human temporal cortex. Neuroscience (2006) 138(2):389–401. 10.1016/j.neuroscience.2005.11.054 [DOI] [PubMed] [Google Scholar]
- 100.Liu X, Bolteus AJ, Balkin DM, Henschel O, Bordey A. GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes. Glia (2006) 54(5):394–410. 10.1002/glia.20392 [DOI] [PubMed] [Google Scholar]
- 101.Pellegrini E, Mouriec K, Anglade I, Menuet A, Le Page Y, Guegeun MM, et al. Identification of aromatase-positive radial glial cells as progenitor cells in the ventricular layer of the forebrain in zebrafish. J Comp Neurol (2007) 501(1):150–67. 10.1002/cne.21222 [DOI] [PubMed] [Google Scholar]
- 102.Djavadian RL. Serotonin and neurogenesis in the hippocampal dentate gyrus of adult mammals. Acta Neurobiol Exp (Wars) (2004) 64(2):189–200. [DOI] [PubMed] [Google Scholar]
- 103.Norton WH, Folchert A, Bally-Cuif L. Comparative analysis of serotonin receptor (HTR1A/HTR1B families) and transporter (slc6a4a/b) gene expression in the zebrafish brain. J Comp Neurol (2008) 511(4):521–42. 10.1002/cne.21831 [DOI] [PubMed] [Google Scholar]
- 104.Pérez MR, Pellegrini E, Nicolau JC, Gueguen MM, LeGuillou DM, Merot Y, et al. Relationships between radial glial progenitors and 5-HT neurons in the paraventricular organ of adult zebrafish-potential effects of serotonin on adult neurogenesis. Eur J Neurosci (2013) 38(9):3292–301. 10.1111/ejn.12348 [DOI] [PubMed] [Google Scholar]
- 105.Klempan T, Hudon-Thibeault AA, Oufkir T, Vaillancourt C, Sanderson JT. Stimulation of serotonergic 5-HT2A receptor signaling increases placental aromatase (CYP19) activity and expression in BeWo and JEG-3 human choriocarcinoma cells. Placenta (2011) 32(9):651–6. 10.1016/j.placenta.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 106.Xing L, Esau C, Trudeau VL. Direct regulation of aromatase b expression by 17β-estradiol and dopamine D1 receptor agonist in adult radial glial cells. Front Neurosci (2016) 9:504. 10.3389/fnins.2015.00504 [DOI] [PMC free article] [PubMed] [Google Scholar]