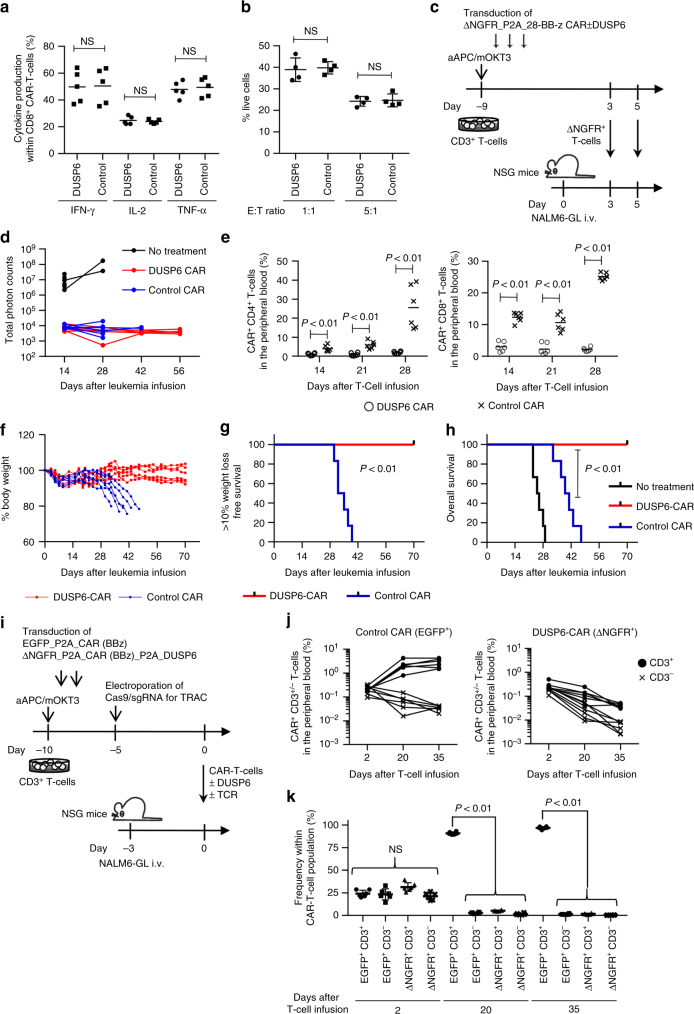
Fig. 6.
Elevated DUSP6 expression in chimeric antigen receptor (CAR)-engineered T cells prevents the development of GVHD, while maintaining the antitumor effects. a, b Human CD3+ T cells were transduced with the anti-CD19 CAR linked with ΔNGFR or ΔNGFR and DUSP6. The cytokine production upon stimulation with K562 cells expressing CD19 (a, n = 5 different donor samples; paired two-sided t-test) and cytolytic activity against the CD19+ NALM6 leukemia cell line expressing the EGFP-luciferase fusion gene (NALM6-GL) (b, n = 4 technical replicates; unpaired two-sided t-test) by the CAR-T cells were evaluated with flow cytometry. c NSG mice were intravenously infused with NALM6-GL (day 0), then with CAR-T cells with or without ectopic expression of DUSP6 on days 3 and 5 (3 million CAR-T cells per infusion). d Total photon counts measured by in vivo bioluminescence imaging (IVIS). e The frequency of human CD45+ CD4+/CD8+ CAR-T cells in the peripheral blood (n = 6 mice for each CAR, unpaired two-sided t-test). f The sequential monitoring of the body weight relative to the weight on day 0. g, h Kaplan–Meier analysis for more than 10% weight-loss-free survival (g) and overall survival (h) after NALM6-GL infusion (n = 6 mice for each group, log-rank test). Representative data of two independent experiments. i–k CD3+ T cells were retrovirally transduced with EGFP-P2A-BB-z CAR or ΔNGFR-P2A-BB-z CAR-P2A-DUSP6, and endogenous TCR expression was ablated using CRISPR/Cas9. NALM6-bearing mice were coinfused with CD3+/− control and DUSP6-CAR-T cells (4 million T cells per mouse). The persistence of CAR-T cells was monitored in peripheral blood. The frequency of each CAR-T-cell population among peripheral blood mononuclear cells (j) or CAR-T cells (k) is shown (n = 6 mice, repeated measures one-way ANOVA with Tukey’s multiple comparisons test). Representative data of two experiments. NS, not significant. Horizontal lines indicate the means ± s.d