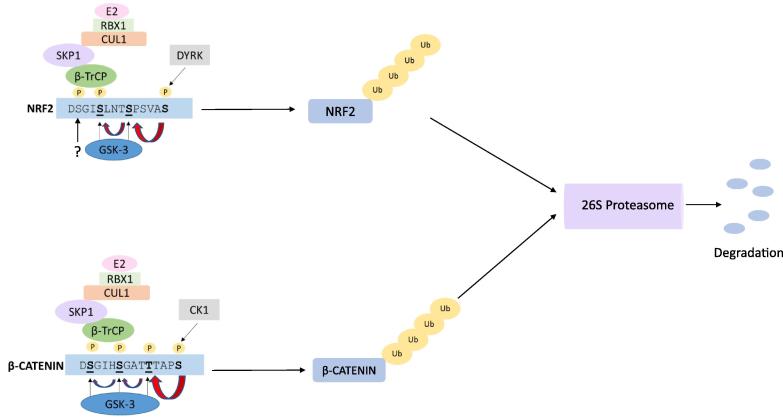
Fig. 1.
Schematic comparison of two proteins which are targeted for degradation in a GSK3 and β-TrCP-dependent fashion. Pharmacological inhibition of GSK3 activity would be predicted to lower recognition by β-TrCP, preventing ubiquitination by CUL1 and co-ordinated stabilization of both proteins. However there is scope for physiological and pharmacological target specific regulation. 1) β-Catenin, but not NRF2, is present within a complex that permits regulation by Wnt signaling, hence GSK3 inhibition by Wnt signaling would stabilize b-catenin but not NRF2, 2) the two proteins are primed by distinct protein kinases providing the possibility for enhancing or reducing GSK3 targeting through regulation of the priming kinase and 3) GSK3 may only phosphorylate one of the two serines in the NRF2 phosphodegron, and a distinct kinase may be needed to fully engage β-TrCP and enhance degradation, while GSK3 is sufficient to complete the β-TrCP-binding motif in β-catenin.