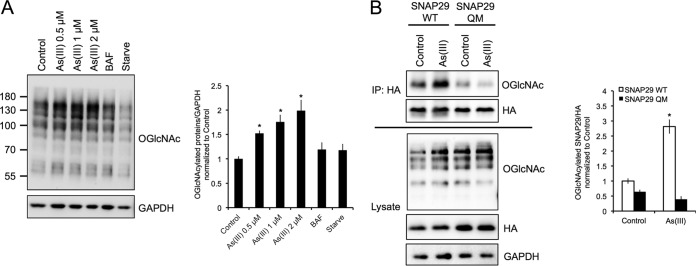
FIG 4.
Arsenic promotes the O-GlcNAcylation of SNAP29. (A) HeLa cells were left untreated or treated with 0.5, 1, or 2 μM As(III), 100 nM BAF, or HBSS for 4 h. Protein O-GlcNAcylation was then assessed via immunoblot analysis of Ser/Thr residue O-GlcNAcylation using the N-acetylglucosamine antibody. GAPDH was used as an internal loading control. Data are means ± SEMs (n = 3). *, P < 0.05 compared to the value for the control (Student's t test). (B) HEK293 cells were transfected with wild-type HA-tagged SNAP29 (WT) or HA-tagged SNAP29 where four key Ser/Thr residues that are O-GlcNAcylated were mutated (SNAP29-QM). Cells were then treated with 0.5 μM As(III) for 4 h and immunoprecipitated using the anti-HA antibody. Equal amounts of HA-SNAP29 were loaded for immunoblot analysis of O-GlcNAcylated SNAP29 using the N-acetylglucosamine antibody. GAPDH was used as an internal loading control. The graph presents the level of O-GlcNAcylated SNAP29 WT versus SNAP29-QM normalized to HA. Data are means ± SEMs (n = 3). *, P < 0.05 compared to the value for the untreated SNAP29 WT control (Student's t test).