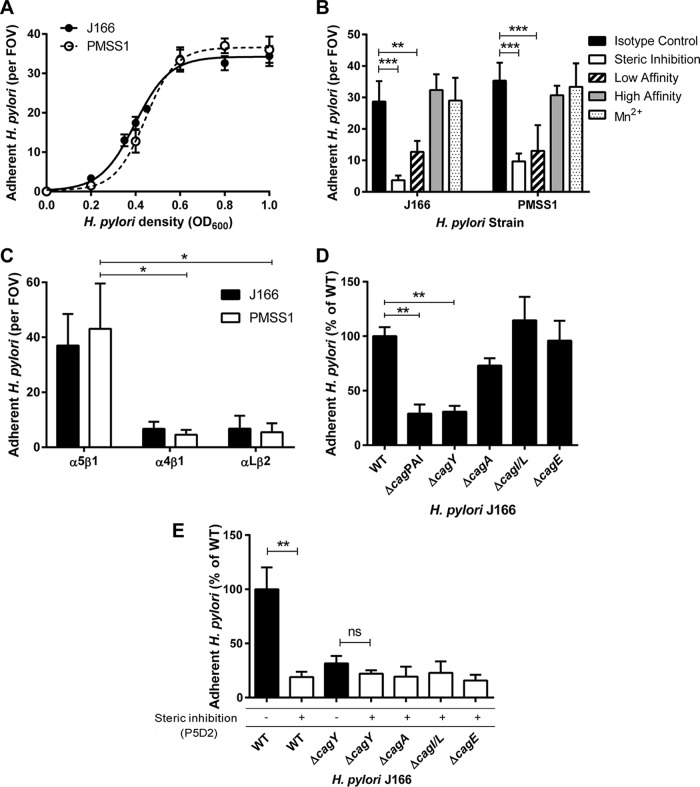
FIG 2 .
α5β1 integrin adherence of WT H. pylori J166 and cagPAI deletion mutants. (A) Adherent H. pylori J166 and PMSS1 cells per field of view (FOV) as a function of bacterial optical cell density at 600 nm (OD600). (B) Adherent H. pylori after preincubation of flow cells with B11/6 isotype control antibody, P5D2 antibody to sterically inhibit β1 integrin binding, or antibodies to lock the integrin in the low-affinity (SG19) or high-affinity (TS2/16) conformation, respectively. Treatment of integrin with Mn2+ to stabilize the high-affinity state produced results similar to treatment with TS2/16 and the B11/6 isotype control antibody. (C) Adherence to α5β1, α4β1, and αLβ2 integrins. (D) Adherence to α5β1 integrin of the J166 WT and deletion mutants, which were fluorescently labeled with DiO and DiI, respectively, mixed in a 1:1 ratio, and enumerated by counting fluorescent bacteria per FOV. Results are expressed as the ratio of deletion mutant to WT. (E) Steric inhibition with P5D2 antibody (white bars) demonstrated that adherence is integrin specific. ΔcagY mutant adherence was similar with and without steric inhibition, suggesting that it represents only nonspecific background binding. Results are the mean ± SEM from 3 to 5 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant.