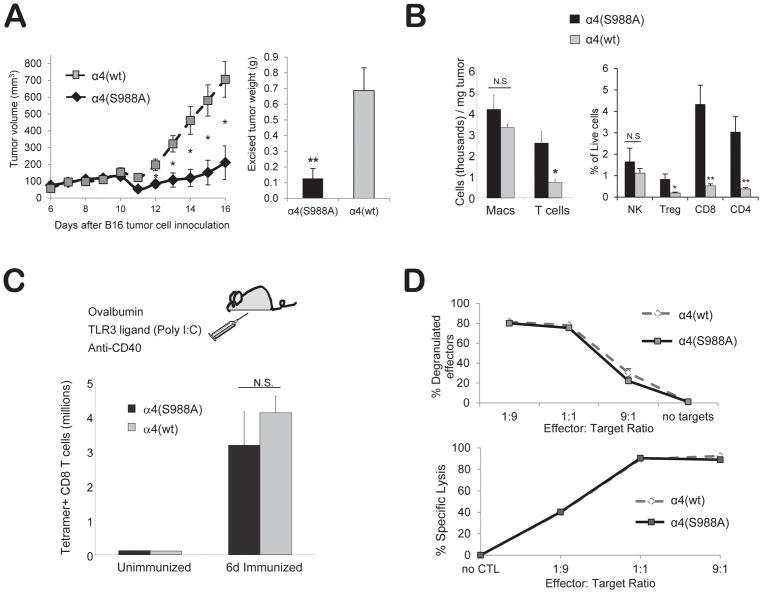
Figure 3.
Blocking α4 integrin phosphorylation increases tumor-infiltrating T-cells and reduces tumor growth in mice. (A) Reduced tumor growth in α4 (S988A) mice. B16 melanoma cells (3×105) were injected into α4(S988A) or control α4(wt) mice. Tumor area was measured daily and converted to an ellipsoid volume: (length x width2)/2. Fifteen days later, tumors were excised and weighed. (B) α4(S988A) T-cells migrate more efficiently to a tumor site. B16 tumors were grown as in (A). On day 15, mice were sacrificed and excised tumors were weighed, digested with collagenase, and stained for (CD45+) CD11b+ macrophages, CD3+ T cells, CD4+ T-cells, CD8+ T-cells, Foxp3+(CD4+) Treg, and NK1.1+ NK cells. Error bars are S.E.M. of n 10 mice per group. * p < 0.015, ** p < 0.002. (C) T-cell clonal expansion. α4(S988A) and α4(wt) control littermate mice were immunized with ovalbumin protein, anti-CD40, and Poly I:C, i.p. in PBS. Six days later, splenocytes were stained with anti-CD8 antibody and an H-2Kb-SIINFEKL tetramer. (D) T-cell cytotoxic function. Splenocytes of 8–12 wk-old OT-I+ α4(S988A) or OT-1+ α4(wt) control littermate mice were differentiated to CTL in vitro with SIINFEKL (1μ/ml) and IL-2 for 5 days and cultured for 2 h at the indicated effector to target ratios (E:T) in the presence of anti-LAMP-1 antibody, followed by staining for CD8 and flow cytometric analysis. For target lysis, CTL were cultured with SIINFEKL-pulsed or unpulsed targets that had been differentially labeled with CFSE. Specific killing was detected by flow cytometry as the decrease in the percentage of specific targets. No significant differences were noted, with the exception of the 9:1 E:T ratio at which the α4(S988A) specific killing was 3.7% lower than α4(wt) CTL (p<.005). n = 3 mice per group. Experiment was performed twice.