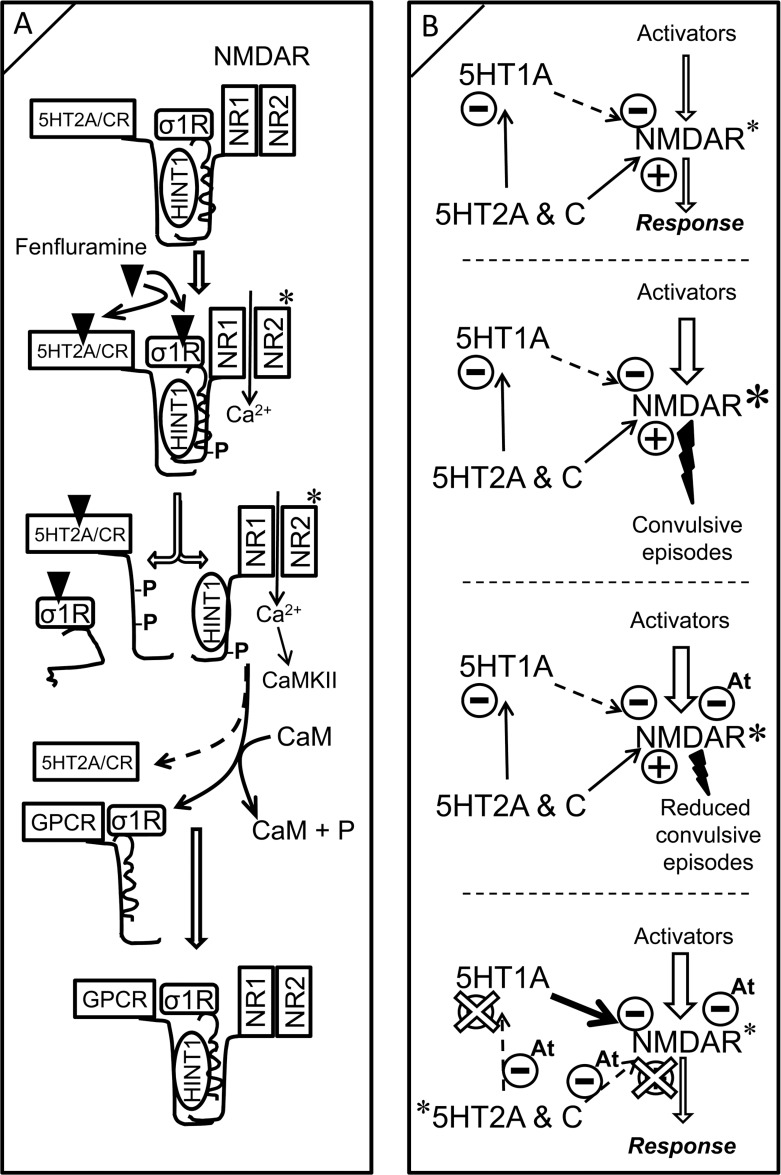
Figure 6. Modeling fenfluramine activity at 5HT2A/C and σ1 receptors. Influence on negative control of NMDAR function by the 5HT1A receptor.
(A) A certain pool of 5HT2A/C receptors is complexed with NMDARs via HINT1 proteins and σ1Rs. Fenfluramine binds and activates 5HT2A/C receptors, promoting PKC/Src priming of the coupled NMDARs. The binding of antagonists such as fenfluramine to σ1Rs prevents σ1Rs from binding to NMDARs [37], enabling HINT1 transfer from the GPCR to NMDAR NR1 subunits. The phosphorylation of 5HT2A/CR cytosolic residues inhibits its re-association with HINT1 to recruit further NMDAR activity. Because HINT1 allows CaM to access NR1 subunits [36], the inactive HINT1-NMDARs can now couple with other GPCRS such as CB1Rs and 5HT1ARs. (B) While 5HT1ARs inhibit NMDAR activity, 5HT2A/CRs promote NMDAR function directly and furthermore, promote it indirectly through the negative control of 5HT1AR signaling. Exaggerated activation of NMDARs may promote seizures. Antagonists of σ1Rs (At) remove σ1Rs from NR1 subunits and enable negative control by CaM; the incidence of convulsive episodes diminishes. The combined activity of fenfluramine as agonist at 5HT2R and antagonist at σ1R uncouples 5HT2Rs from positive control of NMDAR function and increases the inhibitory coupling of GPCRs, such as the 5HT1AR, to NMDAR function. In addition, fenfluramine, as a σ1R antagonist, facilitates CaM inhibition of already primed and GPCR-freed NMDARs. Fenfluramine, by triggering these mechanisms, efficaciously reduces the incidence of epileptogenic episodes. A and B, *indicates activation; B, *indicates higher activity than*.