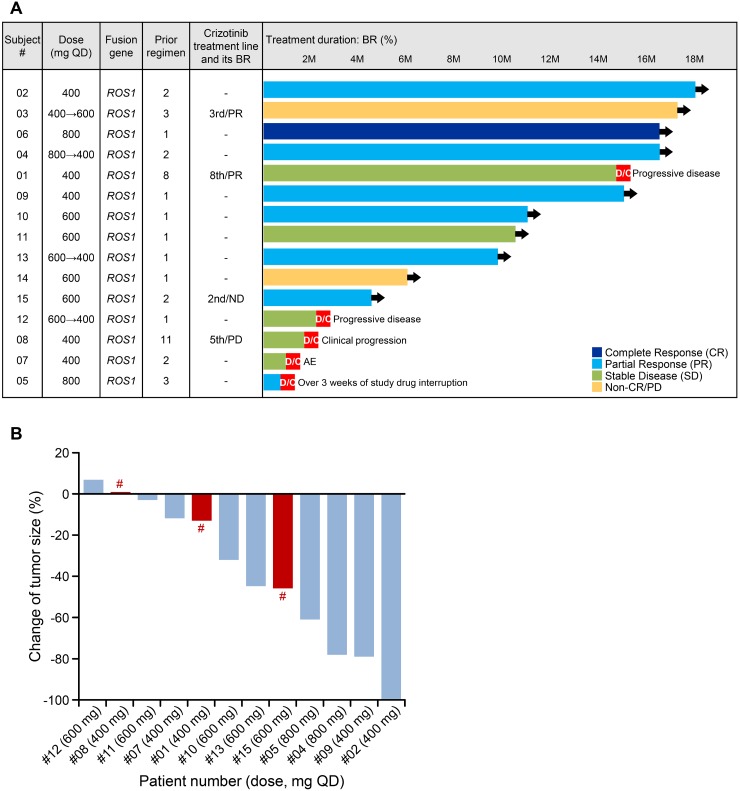
Figure 2. Efficacy endpoints.
Efficacy of DS-6051b (n = 15; cut-off date: 6 July 2017) (A). Best percentage change of tumor size from baseline in patients with target lesion (B). #Crizotinib pre-treated patient. Non-CR/non-PD: persistence of one or more non-target lesion(s) and/or maintenance of tumor marker level above the normal limits; BR, best response; QD, once daily; M, month (4 weeks); D/C: discontinued. Black arrow (➔) indicates ongoing treatment.