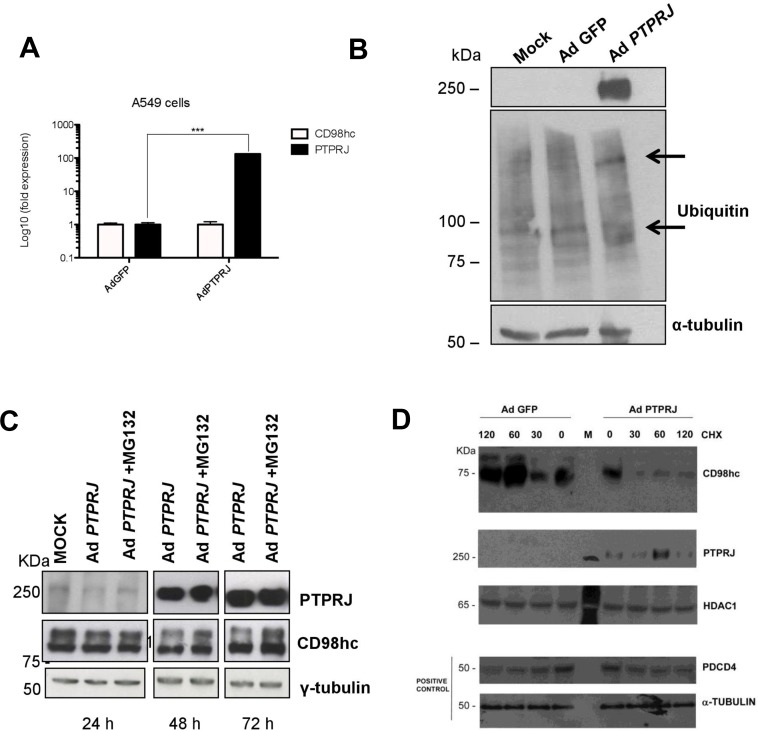
Figure 4. PTPRJ mediates CD98hc stability and proteasome degradation.
(A) A549 cells were seeded in 100 mm culture dishes and, twenty-four hours later, transduced by a recombinant Ad PTPRJ at MOI50. After seventy-two hours, cells were lysed and retro-transcribed mRNA was analyzed by qRT-PCR using specific primers amplifying both CD98hc and PTPRJ. Values were normalized to HPRT RNA levels. *P < 0.05. (B) A549 cells were transduced with Ad PTPRJ at MOI50; cells were lysed and extracted proteins loaded on polyacrylamide gel, transferred to nitrocellulose filter, and analyzed by Western blot through PTPRJ and ubiquitin antibodies. α-tubulin was used to normalize protein loading. (C) PTPRJ-overexpressing A549 cells were lysed after a 6 h treatment with 10 microM MG132, a proteasome inhibitor. The protein extract was analyzed by Western blot analysis evaluating CD98hc levels. γ-tubulin antibody was used to normalize. (D) A549 cells were seeded in 100 mm culture dishes. The cells were transduced by a recombinant Ad PTPRJ or Ad GFP as a control both at MOI50. Fourty-eight hours after infection, cells were treated with cycloeximide 100 μg/mL at different time points. Total proteins were extracted, loaded on polyacrylamide gel and stained with anti CD98hc, PTPRJ and Pdcd4. Equal loading was verified by tubulin and HDAC.