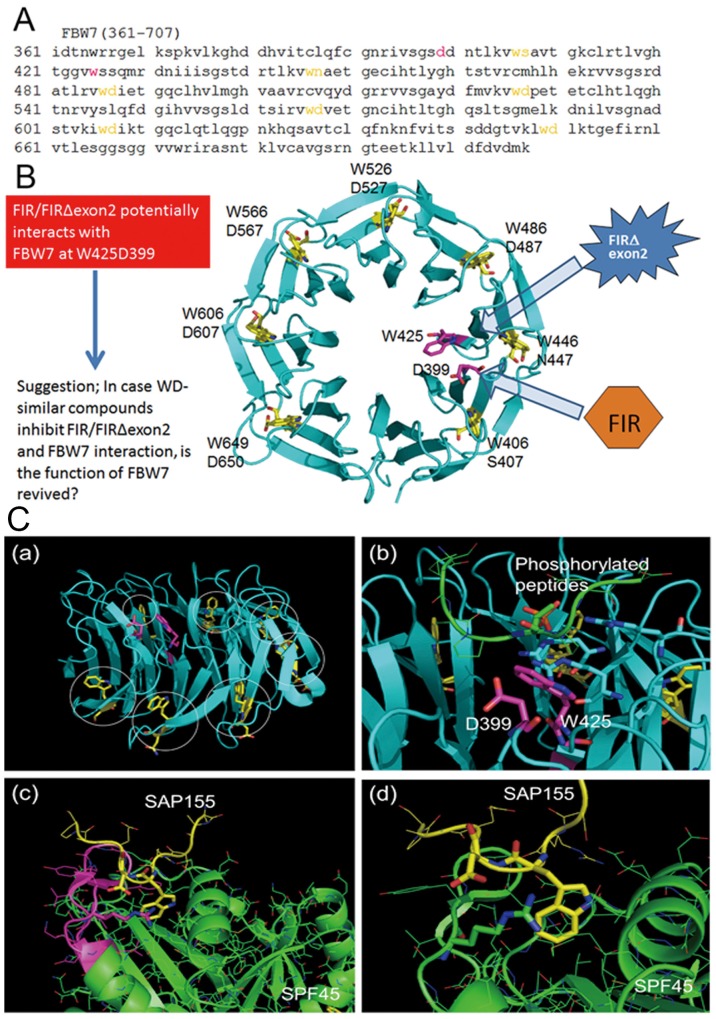
Figure 5. Potential interaction between FBW7 and FIR/FIRΔexon2 revealed by computational three-dimensional crystal structural analysis.
Three-dimensional crystal structural analysis of FBW7 revealed that Trp (W) and Asp (D) were located next to each other in its degron pocket as a WD domain-like structure that possibly bound to the UHM-domain in the carboxyl-terminus of FIR. (A) Amino acids sequence (aa. 361–707) of substrate binding site is indicated. CPD bond propeller pocket in FBW7 also is known as the “degron pocket.” Neighboring W and D (indicated in yellow) potentially binds to FIR-UHM (see text). (B) All neighboring WD domain (indicated in yellow) formed the structural backbone of the “degron pocket” of FBW7. Interestingly, WD-like motifs (W425 and D399) of the “degron pocket” are closely located to each other in 3D structure after protein folding, and it has been suggested that they interact with FIR-UHM (LNGRWFAGRKVVA) (indicated in magenta). (C-a) Structure of CPD of FBW7. WD motifs to stabilize the CPD folding are colored yellow and indicated by circles. W and D residues colored magenta are not involved in the CPD folding. (C-b) Binding of a phosphorylated peptide to CPD. The phosphate group makes an interaction with three R residues shown by the stick representation. The W and D residues not involved in the CPD folding are positioned at the middle of CPD. (C-c) Interaction of SAP155 and SPF45. The LNGRYFGGRVVKA motif of SPF45 is colored magenta. (C-d) Close view of the SAP155-SPF45 interaction. W and D residues of SAP155 shown in yellow made a strong interaction with R304 of SPF45, depicted in the stick representation.