ABSTRACT
An epigenetic transition between white cells and opaque cells influences several properties of Candida albicans biology, including cellular morphology, biofilm formation, virulence, and sexual mating. In particular, these two cell types exhibit marked differences in their ability to undergo sex. A previous study identified the transcriptional regulator of pheromone response in both the white and opaque states as Cph1 because deletion of this gene abolished both pheromone-induced cell adhesion in white cells and sexual mating in opaque cells. To further explore how these cell types exhibit distinct biological outputs upon pheromone stimulation, we selected five Cph1-regulated genes with significant expression during the pheromone response in the white state but not the opaque state. These phase-specific pheromone-induced genes are ORF19.1539, ORF19.1725, ORF19.2430, ORF19.2691 and ORF19.5557. Deletion of each gene revealed that orf19.1539Δ, orf19.1725Δ, orf19.2430Δ and orf19.5557Δ showed significant decreases in pheromone-stimulated cell adhesion in the white state but retained normal mating competency in the opaque state, indicating that a particular role in white cell pheromone response is mediated by these four genes. Interestingly, the defects of orf19.1725Δ in pheromone-stimulated cell adhesion also abolished conventional biofilms and hyphal growth. Zebrafish egg infection assays further demonstrated that ORF19.1725 is involved in cell adhesion, penetration and virulence. Overall, four Cph1-regulated downstream targets were identified in the regulation of white cell pheromone response. We also clarified the roles of C. albicans ORF19.1725 in cell adhesion, hyphal growth, biofilm formation and virulence.
KEYWORDS: Candida albicans, opaque cells, ORF19.1725, pheromone response, virulence, white cells
Introduction
Candida albicans can be a harmless, normal member of the microbiota in and on the human body [1]. However, it is also an opportunistic pathogen that can attack multiple locations in a human host, including the skin, genitals, mucous membranes and kidneys, possibly resulting in life-threatening systemic infection or invasive candidiasis [2,3]. The propensity of C. albicans to adapt and become pathogenic in different niches has been closely linked to its phenotypic plasticity [4,5]. Indeed, several diverse C. albicans cell types, including yeast cells, hyphal cells, pseudohyphae, chlamydospores, GUT (gastrointestinally induced transition) cells, gray cells and opaque cells [6-9], demonstrate the ability of Candida cells to alter their morphology and behavior in response to environmental signals.
Among these cellular alterations, the reversible morphological transition between white cells and opaque cells is particularly interesting, because these two morphologically distinct cell types exhibit a wide range of different biological behaviors. Typically, white cells are mating incompetent, can release immune-cell chemoattractants and exhibit greater virulence in systemic infections, whereas opaque cells can be fertilized and cause more serious infections of mammalian skin [10-12]. Furthermore, white and opaque cells produce different sets of chemoattractants, thereby affecting C. albicans interactions with phagocytic cells [13–18]. Specifically, white cells release Sap2, Sap6 and an undetermined low-molecular-mass peptide as chemoattractants and are recognized by phagocytes, whereas opaque cells produce Sap1, Sap2 and Sap3 and are able to escape phagocytosis [13,14,18]. Additionally, transcriptional profiling has shown that white cells and opaque cells display large-scale differences in the expression of metabolic genes [19,20]. Indeed, a very recent report has shown that the white cell state is an “intrinsic phenotype”, allowing this cell type to grow better at high temperatures (37°C) in mammalian hosts and in response to many nutritional conditions and chemical stresses [21]. However, opaque cells show greater fitness than while cells under poor nutrition conditions and in certain environmental circumstances [21]. Despite the higher fitness of white cells under a wide range of environmental conditions, several environmental stimuli, including oxidative stress, CO2 and N-acetylglucosamine (NAG), induce opaque cell formation [22–24].
The regulation of epigenetic switching between the white and opaque states in C. albicans involves a complicated interlocking transcriptional feedback loop of eight factors, Wor1, Wor2, Wor3, Wor4, Czf1, Efg1, Ahr1 and Ssn6 [19,20,25–29]. This network is also controlled by the a1/α2 heterodimer protein generated by a/α cells, which leads to the repression of white-to-opaque switching [30].
One of the most interesting differences between white cells and opaque cells is their behavior during pheromone response, in which, unlike opaque cells, white a or α cells do not undergo a mating response but display increased cell adhesion (pheromone-stimulated cell adhesion) and sexual biofilm development [31,32]. In addition, it has been suggested that sexual biofilm formation by white cells provides an optimal condition for opaque cell mating [33]. Genetic analyses have defined the mechanism by which pheromone signaling activates cellular responses in white and opaque cells. In particular, both cell types, when challenged with pheromone, are regulated by the same signaling pathway and the same transcription factor, Cph1 [31]. A key question, then, is how C. albicans displays distinct functions upon exposure to the same pheromone signal while still utilizing the same transcription factor. It is possible that some downstream genes regulated by Cph1 may be different in white and opaque cells after pheromone treatment, leading to different responses in these two cell types. Interestingly, Cph1 is not required for the formation of “conventional biofilms” [31]. Conventional biofilms, which are chiefly regulated by six transcription factors in C. albicans (Tec1, Efg1, Rob1, Ndt80, Bcr1 and Brg1) [34], are formed when C. albicans yeast cells adhere to a surface, form pseudohyphae and hyphae and produce extracellular matrix materials [35,36]. Thus, the genetic control of these two distinct biofilm types in C. albicans is mediated by different mechanisms [31,32].
In this study, we used previous transcriptional profiling data [31] to identify five novel downstream genes that are highly regulated by Cph1 in the C. albicans white state when challenged with pheromone but are less regulated or unaffected by Cph1 and pheromone challenge in the opaque state. We therefore hypothesized that these genes might play a specific role in white cell pheromone response. Deletion of each gene revealed that most of the mutant strains showed effects on pheromone-stimulated cell adhesion in white cells. In particular, orf19.1539Δ, orf19.1725Δ, orf19.2430Δ and orf19.5557Δ showed significant reductions in cell adhesion during pheromone response. Interestingly, ORF19.1725 is also involved in virulence-associated functions, including the formation of conventional biofilms, hyphal formation, cell adhesion and virulence. Overall, our data reveal novel genes that specifically contribute to white cell pheromone response. These results provide a clue to the mechanism that allows white and opaque cells to display distinct pheromone responses and behaviors.
Results
Screening for white-specific and Cph1-regulated genes in pheromone-challenged P37005
Transcriptional profiling has shown that many pheromone response genes are identical in both white and opaque cells [31]. To further explore the regulatory differences between the white and opaque states during pheromone response, we screened genes that were significantly upregulated by Cph1 in white cells. Five highly expressed genes in the white state showed significant differences (P < 0.05) in expression fold change between white cells and opaque cells of the wild-type strain during the response to α-pheromone [31]. These genes were ORF19.1539, ORF19.1725, ORF19.2430, ORF19.2691 and ORF19.5557 (MNN4). To confirm these results, quantitative PCR was performed. As shown in Fig. 1, all candidate genes were highly expressed during pheromone response in the white state. However, the expression level of each gene was significantly reduced in the P37005 cph1Δ strain after pheromone treatment. These results showed that Cph1 is a key regulator and is required for the expression of these five genes in white cell pheromone response. Among them, only MNN4 has a known function, and it has not been studied in depth; this gene encodes a mannosyltransferase and is involved in the cell wall formation and engulfment process during phagocytosis in C. albicans [37–39]. The functions of the other selected genes are entirely unknown, and none have been studied previously.
Figure 1.
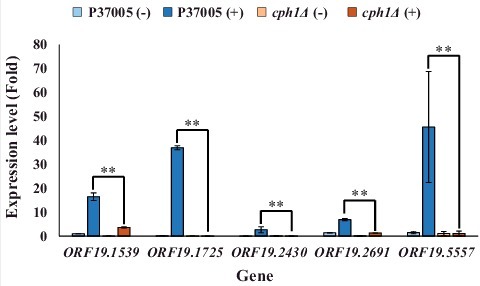
Quantitative RT-PCR demonstrated that expression of the ORF19.1539, ORF19.1725, ORF19.2430, ORF19.2691 and ORF19.5557 genes in white cell pheromone response were regulated by Cph1 in P37005. Expression was analyzed and compared between the wild-type and cph1Δ with or without α-pheromone peptide treatment. Expression was normalized to that of the ACT1 gene. Values are the mean ± SD of three experimental replicates, and two technical repeats were performed for each experimental replicate. **: P < 0.01.
orf19.1539Δ, orf19.1725Δ, orf19.2430Δ and orf19.5557Δ reduced cell adhesion ability in white cells during pheromone response but were dispensable in cellular mating of opaque cells in P37005
To understand their functions in pheromone response, each gene was deleted by using a target recombination strategy, as described in the Materials and Methods. Then, the pheromone response of white cells was investigated to see whether the deletion strains were defective in cell adhesion. Fig. 2A shows that compared to the P37005 wild-type strain, the adherent numbers of orf19.1539Δ, orf19.1725Δ, orf19.2430Δ and orf19.5557Δ were 6.2 × 107, 6.4 × 107, 7.0 × 107 and 7.8 × 107, respectively, significantly lower than that of wild-type strain P37005 (9.47 × 107). Pheromone responses between the white and opaque states are typically distinct. Opaque cells mate; white cells, however, generate a sexual biofilm and promote cell adhesion [31–33]. To further determine whether these mutant strains defective in white cell pheromone response also showed phenotypes in opaque cell pheromone response, mating assays were performed. Interestingly, the orf19.1539Δ, orf19.1725Δ, orf19.2430Δ and orf19.5557Δ strains displayed normal a-α mating efficiencies (Fig. 2B). These results indicated that these four genes are specifically upregulated in C. albicans white cells in order to execute precise biological responses.
Figure 2.
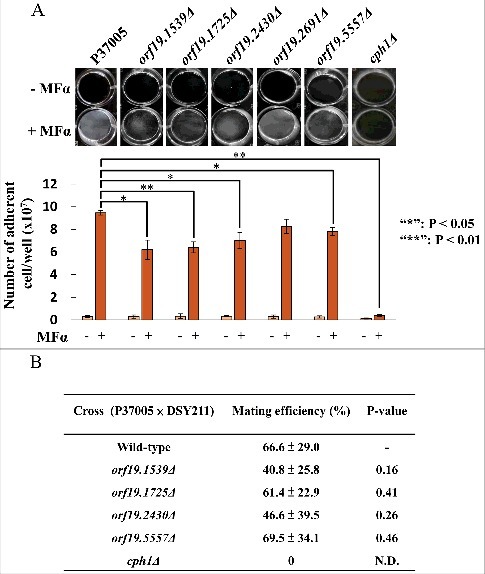
ORF19.1539, ORF19.1725, ORF19.2430 or ORF19.5557 in C. albicans P37005 are specifically involved in white cell pheromone response but are dispensable in opaque cell pheromone response. (A) White cells of P37005 cultured in Lee's medium in the presence or absence of pheromone (MFα) in plastic dishes. Images were taken after PBS washing (top panel), and the remaining adhered cells were quantitated (bottom panel). (B) Opaque cells of the wild-type and mutant strains (MTLa/a; SAT−, Arg+) were crossed with the MTLα/α DSY211 strain (SAT+, Arg−) on Spider medium for 48 hr. Cells were plated onto selective media (SAT+, Arg−) to quantitate mating efficiencies. The cph1Δ strain of P37005 served as a negative control. Values are the mean ± SD of three experimental replicates, and two technical repeats were performed for each experimental replicate. *: P < 0.05; **: P < 0.01.
ORF19.1725 is required for conventional biofilm development
Previous works have demonstrated that Cph1 is not involved in conventional biofilms [31]. Instead, the conventional biofilm regulatory network is modulated by Tec1 and five other major transcription factors [34]. Loss of any one of these regulators significantly compromises biofilm formation in vitro and in vivo [34]. To further investigate the five candidate white-specific pheromone response genes in P37005, we also tested their roles in conventional biofilm development. Among them, one gene significantly influenced biofilm formation on silicone, ORF19.1725, as deletion of this gene led to a biofilm mass of 1.3 mg, whereas the biofilm mass of wild-type strain P37005 was 4.95 mg (Fig. 3A). This result agrees with previously published microarray data, in which ORF19.1725 was regulated by both Cph1 and Tec1 [31,40]. Indeed, quantitative RT-PCR confirmed the regulation of ORF19.1725 by Tec1 (Fig. 3B), a transcription factor required for the formation of conventional biofilms but not for pheromone-stimulated cell adhesion [31,34].
Figure 3.
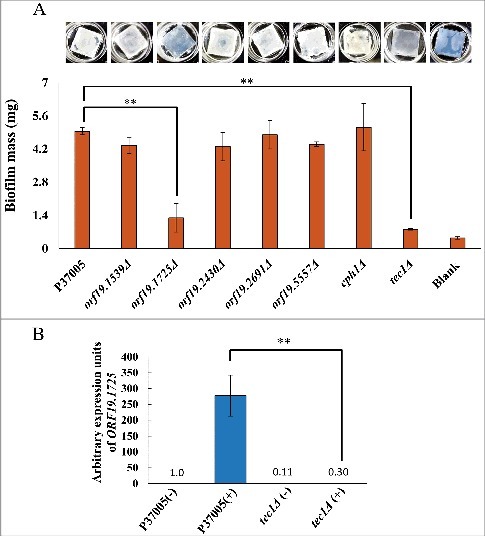
ORF19.1725 is also regulated by Tec1 and is required for the formation of conventional biofilms. (A) Analysis of five candidate genes in a conventional biofilm assay on silicone squares revealed that the presence of the ORF19.1725 gene is necessary for the development of conventional biofilms. The graph shows the mean ± SD of three experimental replicates, and two technical repeats were performed for each experimental replicate. “**”: P < 0.01. (B) Quantitative RT-PCR revealed that ORF19.1725 expression was regulated by Tec1. Expression was analyzed and compared between the wild-type and tec1Δ in response to pheromone treatment. Expression was normalized to that of the ACT1 gene. Values are the mean ± SD of three experimental replicates, and each replicate represents two technical repeats. “**” represents P < 0.01. – and + indicate with or without pheromone treatment, respectively.
ORF19.1725 deletion caused a severe defect in hyphal formation in C. albicans P37005
We have shown that the presence of ORF19.1725 is important for biofilm formation in C. albicans P37005. Moreover, our data showed that genetic control of ORF19.1725 is mediated by two regulators, Cph1 and Tec1. TEC1 deletion strains in C. albicans produce no biofilms and are defective in hyphal development [41]. We then examined whether ORF19.1725 also plays a role in hyphal formation. As expected, removing ORF19.1725 caused a significant hyphal development deficiency when cultured in 50% serum (Fig. 4A). Additionally, after 12 hr of culture in serum, both the wild-type and complemented strains displayed obvious cell accumulations and clumps (hyphal formation; Fig. 4B), whereas orf19.1725 mutants did not. These results indicate that ORF19.1725 is involved in hyphal development. In order to determine whether or not growth defects happen in ORF19.1725 deletion strains, thereby causing a severe deficiency in hyphal development, growth curves of the wild-type, mutant and complementation strains were determined. Fig. 4C shows that orf19.1725 mutant strains did not exhibit growth defects.
Figure 4.
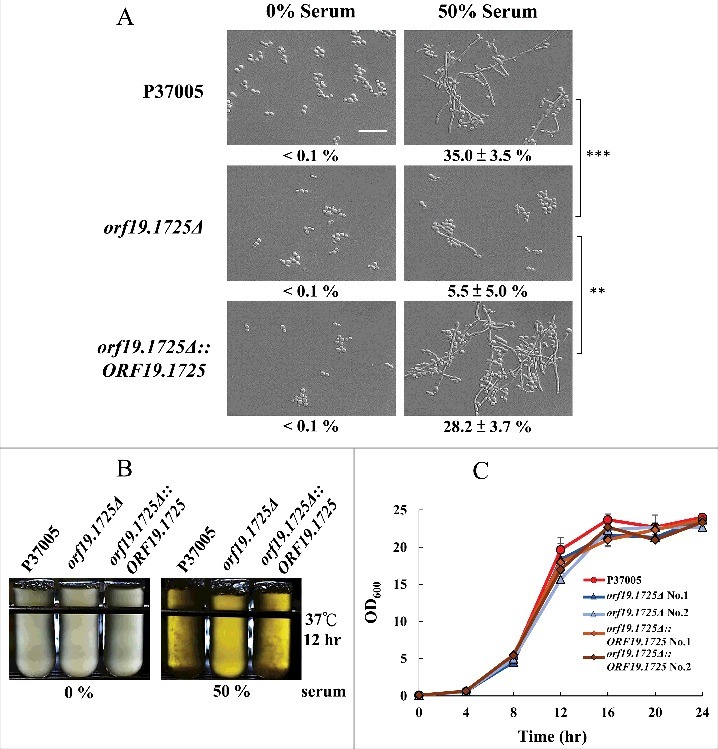
The orf19.1725Δ strain was defective in hyphal formation. (A) Cells of the wild-type, orf19.1725Δ and complemented strains were grown in YPD medium with or without 50% bovine serum. The representative images show that ORF19.1725 gene deletion caused a severe defect in hyphal development. Four fields were checked, and at least 100 cells were counted in each field of every C. albicans strain. Ratios of hyphae formation are displayed below each image. “**” represents P < 0.01 and “***” represents P < 0.001. (B) The wild-type and the complemented strains clearly exhibited clumps after 12 hr of culturing in YPD liquid media at 37°C, whereas the orf19.1725Δ strains did not. Hyphae were observed and examined under a light microscope after the clumps had been collected by centrifugation. Left panel: without serum treatment; right panel: YPD supplemented with 50% bovine serum. Scale bar: 50 μm. (C) Growth curves of C. albicans strains at 37°C. Overnight cultures of YPD-grown C. albicans cells were diluted to an OD600 of 0.1 in fresh YPD liquid media. Growth rates were monitored every 4 hr using a Biowave density meter. Values are the mean ± SD of three experimental replicates, and two technical repeats were performed for each experimental replicate. “**” represents P < 0.01 and “***” represents P < 0.001.
ORF19.1725 potentially encodes an adhesin protein
The major fungal adhesins are glycosylphosphatidylinositol (GPI)-anchored proteins [42]. The primary structure of these GPI-anchored proteins can be divided into three conserved features [42, 43]. The N terminus contains a signal peptide for localization to the endoplasmic reticulum (ER). Although the middle regions of adhesin proteins are diverse, they are often rich in serine/threonine residues and contain tandem repeats. The hydrophobic C-terminal domain contains an ω site, where the protein is cleaved and replaced with a preformed GPI anchor in the membrane of the ER. Orf19.1725 has been predicted to function as an adhesin protein (FungalRV; http://fungalrv.igib.res.in/immuno.html) [44], and the coding region of Orf19.1725 was therefore further analyzed. A signal peptide (SP) in the N-terminal region was predicted (value: 0.452) using SignalP 4.1 with the default cutoff value (0.450) (http://www.cbs.dtu.dk/services/SignalP/) [45], and a cleavage site was predicted between the 26th and 27th amino acids (Fig. 5A and 5B). Although an ω site at the position of the 700th amino acid was predicted by the PredGPI website (http://gpcr.biocomp.unibo.it/predgpi/), this prediction is less likely to be accurate due to a very low specificity (32.5%) (Fig. 5A and 5B) [46]. Interestingly, four tandem repeats were found (Fig. 5A and 5C): TR1 (26 aa), TR2 (17 aa), TR3 (40 aa) and TR4 (55 aa) (http://weblogo.threeplusone.com/create.cgi) [47]. These data reveal that Orf19.1725 might be an adhesin and is involved in cell adhesion. However, the protein sequence of Orf19.1725 does not share any sequence or feature similarities with the three known adhesin families (ALS, HWP and IFF) in C. albicans [42,48–50].
Figure 5.
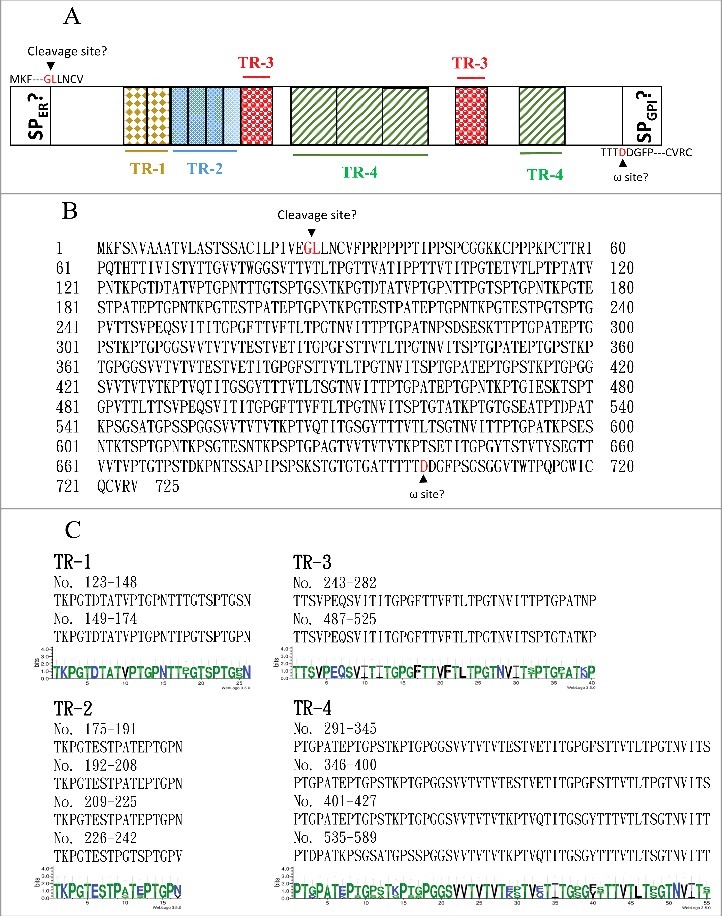
The C. albicans Orf19.1725 protein potentially functions as an adhesin. (A) Analysis of Orf19.1725 reveals three conserved features, a putative signal peptide, four different tandem repeats (TR1, TR2, TR3 and TR4) and a hypothetical ω site. (B) Protein sequence of C. albicans orf19.1725. The arrow between the 26th (Gly) and 27th (Leu) amino acids indicates the likely cleavage site for the signal peptide (http://www.cbs.dtu.dk/services/SignalP/). The other, at the position of the 700th amino acid (Asp), was predicted to be an ω site with lower specificity (http://gpcr.biocomp.unibo.it/predgpi/). (C) Sequence motifs of four different tandem repeats of Orf19.1725 (http://weblogo.threeplusone.com/create.cgi).
Orf19.1725 is required for cell adhesion in a zebrafish egg infection model
To test whether ORF19.1725 has a role in adhesion, cell adhesion assays using a zebrafish egg infection model were performed (Chen et al., 2015). As shown in Fig. 6A, two independent orf19.1725 mutant strains almost entirely failed to attach to zebrafish eggs after 4 hr of inoculation (Fig. 6Ab, c, h, I), whereas the P37005 wild-type (Fig. 6Aa, g) and the complemented strains (Fig. 6Ad, e, j, k) colonized the entire surfaces of the embryo eggs successfully and formed hyphae. Furthermore, the P37005 wild type (Fig. 6Am) and the complemented strains (Fig. 6Ap and q), but not the mutant strains (Fig. 6An and o), were able to penetrate and invade the embryo eggs within the early infection stage (4-hr inoculation). This phenomenon could be caused by the presence of fewer adherent cells in orf19.1725Δ or by defects in hyphal formation in these mutant strains. Infected embryo eggs were then disrupted in a vortex shaker using glass beads to quantitate the adhered cells. The number of adhered cells of the orf19.1725 mutant strains was significantly lower than those of the wild-type and complemented strains (Fig. 6B).
Figure 6.
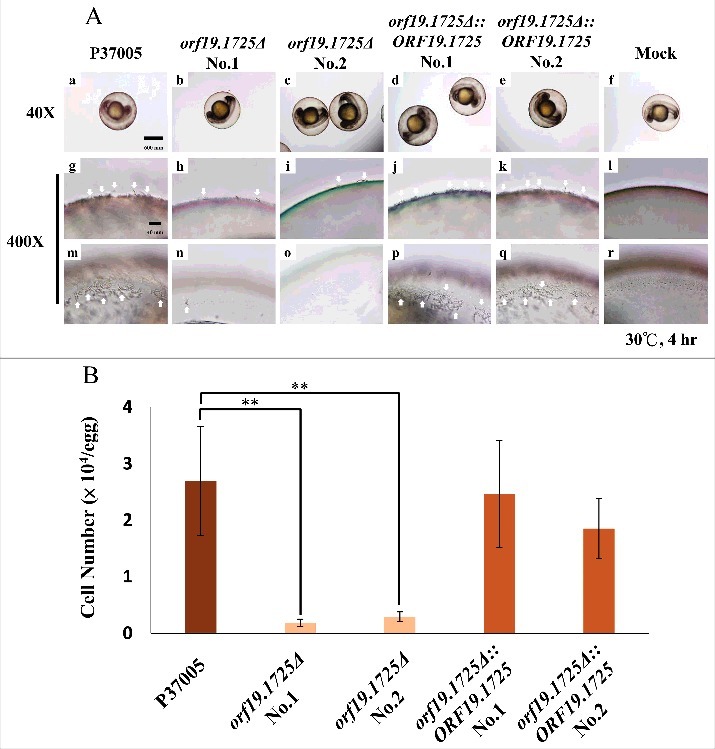
ORF19.1725 is involved in cell adhesion to zebrafish embryos. (A) Embryos were co-incubated with 5 × 105 C. albicans cells of P37005 (a, g, m), orf19.1725Δ (b, c, h, i, n, o) and orf19.1725Δ::ORF19.1725 (d, e, j, k, p, q) for 4 hr. The infected embryos were then washed with egg water to remove non-adhered cells. Images were taken by a Leica TCS SP5 II inverted microscope. The images in the middle panel (g, h, i, j, k, l) are focused on the outside of the embryo surface. The images in the bottom panel (m, n, o, p, q, r) are focused on the inner side of the embryo surface. Embryos without infection by C. albicans cells served as negative controls (f, l, r). White arrows indicate hyphae on or in the embryo surfaces. (B) Cell quantitation revealed a significantly lower number of adhesive cells of orf19.1725Δ on zebrafish embryo surfaces than of the wild-type or the complemented strains. Infected embryos were washed twice with 40 ml of egg water to remove non-adhered cells. The embryo eggs were then disrupted by a FastPrep-24 instrument (MP Biomedicals, Illkirch, France) and plated on YPD plates to quantitate the number of adhered cells. Values are the mean ± SD of three experimental replicates, and two technical repeats were performed for each experimental replicate “**” represents P < 0.01.
ORF19.1725 gene deletion significantly reduced fungal virulence in P37005
We have shown that the orf19.1725Δ strain had a defect in hyphal formation under serum treatment and decreased adhesion ability on zebrafish embryos. To further evaluate the role of ORF19.1725 in virulence, the survival rates of zebrafish embryos at 24 and 48 hr post-inoculation were determined. As shown in Fig. 7A, ORF19.1725 deletion strains resulted in higher embryo survival rates at 24 hr (95.1% and 81.9%) and at 48 hr (53.1% and 61.1%) after infection. In contrast, the P37005 wild-type (61.0%) and the complemented strains (41.5% and 38.4%) had lower embryo survival than that of orf19.1725Δ at 24 hr. Few embryos survived after inoculation with the wild-type (10.9%) and complemented strains (14.9% and 5.8%) at 48 hr. Although microscopy showed that orf19.1725Δ had normal hyphal development on infected embryos at 48 hr (Fig. 7Bb, c, h, i), similar to that of the wild-type (Fig. 7Ba, g) and complemented strains (Fig. 7B d, e, j, k), the ORF19.1725 deletion strains showed remarkable defects in penetration ability (Fig. 7Bb, c, n, o). The inner sides of the embryo surfaces showed that few hyphae had penetrated (Fig. 7Bn, o), leading to a clearer background (Fig. 7Bb, c) in the embryos infected with orf19.1725Δ, whereas the hyphae produced by the wild-type (Fig. 7Ba, m) and complemented strains (Fig. 7Bd, e, p, q) had successfully invaded the embryos.
Figure 7.
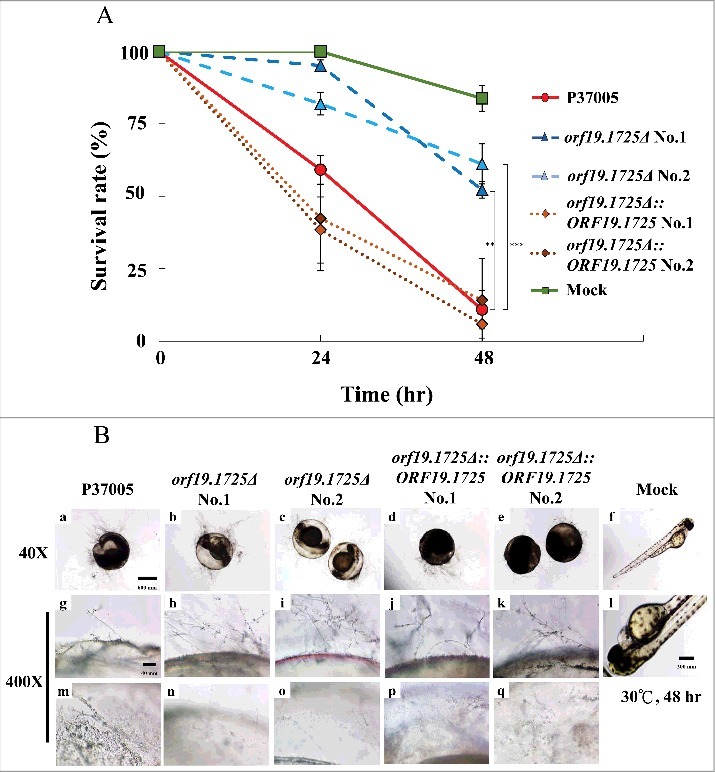
ORF19.1725 deletion strains had reduced virulence. (A) Survival curves of zebrafish embryos during infection with C. albicans cells revealed that Orf19.1725 is involved in pathogenicity. Twenty embryos in each plastic well were co-incubated with 5 × 105 C. albicans cells. Infected embryos were washed and transferred to sterilized egg water. Survival rate was determined after calculating the number of infected zebrafish embryos that retained a heartbeat divided by the total embryo number. Values are the mean ± SD of three experimental replicates, and each replicate represents two technical repeats. “**” represents P < 0.01 and “***” represents P < 0.001. (B) Representative images of embryos infected by C. albicans after 48 hr revealed that orf19.1725Δ exhibited slower infection progress, less penetration ability and a clearer embryo background (b, c, h, i, n, o) than the wild-type strain (a, g, m) and the complemented strains (d, e, j, k, p, q) did. Embryos without infection by C. albicans cells served as mock controls (f, l) and hatched into newborn zebrafish. The images in the middle panel (g, h, i, j, k, l) are focused on the outside of the embryo surface. The images in the bottom panel (m, n, o, p, q, r) are focused on the inner side of the embryo surface.
Discussion
The epigenetic transition between white cells and opaque cells is a unique phenomenon in C. albicans [8,19,20]. White cells and opaque cells not only display distinct cellular appearances and sizes but also exhibit a wide variety of changes in different aspects of their biology, including virulence, biofilm formation, fungus-host interactions and mating [5,10,15,17,31–33,38]. In particular, the pheromone responses in white and opaque cells are behaviorally distinct, in that opaque cells mate, whereas white cells do not mate but instead initiate cell adhesion [31]. Strikingly, expression profiling of C. albicans strain P37005 revealed that the components required for pheromone signaling have significant overlap with the genes regulated by Cph1 in both the white and opaque states [31]. Hence, the strategies of these two distinct cell types of C. albicans to determine a specific regulatory program to respond to pheromone challenge represent an interesting and crucial question.
Three potential assumptions might account for the distinct biological outputs of pheromone-stimulated white cells and opaque cells. (1) Although the overlap in gene expression between pheromone-stimulated white cells and opaque cells was significant, expression levels were higher overall in opaque cells than in white cells. These data imply that gene expression level might be a crucial factor in phenotype-specific response [31]. (2) Furthermore, differential expression levels might play a role in signaling specificity. Indeed, a recent article has shown that co-expression of STE4, CST5, and CEK2 in white cells significantly increased their abilities to undergo cell-cell mating [31]. (3) In S. cerevisiae, differential Ste12-bound complexes are well known to modulate different developmental fates through the MAP kinase pathway [51,52]. During filamentation, the formation of a Ste12-Dig1-Tec1 complex is required, whereas inhibition of the Tec1 transcription factor is necessary to ensure that the sexual program occurs [51,52]. Thus, different Cph1-biding partners likely contribute to target gene binding specificity, leading to different biological responses in C. albicans white cells and opaque cells.
In this study, five genes with significantly higher expression levels in white cells than in opaque cells were selected for further investigation. Four of these five genes, ORF19.1539, ORF19.1725, ORF19.2430 and ORF19.5557, are specifically involved in the pheromone response in white cells, as deletion of each gene significantly reduced pheromone-stimulated cell adhesion but did not affect normal mating ability. These results demonstrate for the first time that a particular set of genes regulated by Cph1 is specifically involved in the white cell pheromone response and differentiates that response from the opaque cell pheromone response.
Genetic control of pheromone-stimulated cell adhesion and conventional biofilm formation in C. albicans is mediated by different mechanisms. In particular, Cph1 is required for pheromone-induced cell adhesion in white cells [31], whereas six transcriptional regulators (Bcr1, Brg1, Efg1, Rob1, Ndt80 and Tec1) have been shown to operate in the biofilm regulatory network [34]. Although Bcr1, Brg1, Rob1 and Tec1 play general roles in mediating pheromone-stimulated cell adhesion as well as in biofilm development [31,32,34], CPH1 deletion strains in C. albicans produce conventional biofilms normally but are completely deficient in pheromone-stimulated cell adhesion [31]. We have previously shown that Hgc1, a G1 cyclin-related protein regulated by both Cph1 and Tec1, is important for both pheromone-stimulated cell adhesion and biofilm development [31]. Here, we have shown that ORF19.1725 is also involved in white cell pheromone response and plays a critical role in conventional biofilm formation in C. albicans P37005. Indeed, ORF19.1725 is also regulated by Tec1. Cells lacking ORF19.1725 exhibit a marked defect in hyphal formation, which may explain the inability of orf19.1725Δ to form conventional biofilms.
Sequence analyses showed that Orf19.1725 might function as an adhesin protein. The results of zebrafish egg adhesion assays demonstrated that orf19.1725Δ had reduced adhesive ability on the surfaces of zebrafish embryos. However, expression of ORF19.1725 fused with green fluorescent protein (GFP) at either the N- or C-terminal end failed to determine its subcellular localization (data not shown). As described previously, an adhesin protein has an SP for entry into the ER and a cleavage ω site for anchoring to a preformed GPI [42,43]. Thus, the GFP fused with the N- and C-terminal sites of Orf19.1725 was likely removed during processing. Insertion of this fluorescent marker into the signal cleavage site or the ω site might be a better strategy to determine its localization. However, whether the SP cleavage site at the N terminus and the ω site at the C terminus of Orf19.1725 are accurate in C. albicans needs to be further addressed, given that the prediction values for both sites were low.
Our results have shown that ORF19.1725 is required for hyphal development and biofilm formation in vitro as well as cell adhesion in vivo. As expected, ORF19.1725 deletion also resulted in a significant decrease in virulence. However, hyphal development and hyphal length between the wild-type and orf19.1725Δ strains were indistinguishable in the zebrafish egg infection model at 48 hr post-inoculation. The distinct hyphal development phenotypes of orf19.1725Δ observed in vitro and in vivo might be due to different environmental conditions. In fact, a variety of hyphal stimulators, such as high temperature, serum, neutral pH and nutrient limitation can promote the yeast-to-hyphae transition through different signaling pathways and transcription factors [7, 53–56].
Despite utilizing the same signaling cascade and transcription factors, white cells and opaque cells exhibit distinct biological outputs in response to pheromone stimulation. Here, we have shown that some pheromone-induced white-specific genes contribute to the phase-specific phenotype of pheromone-responding white cells in C. albicans P37005. Notably, deletion of ORF19.1725 resulted in decreased pheromone-induced cell adhesion in pheromone-responding white cells and abolished biofilm formation, similar to tec1Δ and hgc1Δ [31,41]. Additionally, we have also characterized and determined new roles of C. albicans ORF19.1725 in cell adhesion, hyphal formation and virulence. Most importantly, the results outlined in this study might provide a clue as to how the molecular switch regulates the alternative phenotypic outputs of white cells and opaque cells during pheromone response, with direct consequences for pathogenesis and sex.
Materials and methods
Media and reagents
The media used in these experiments, including yeast extract-peptone-dextrose (YPD), Spider medium and Lee's medium, were prepared as previously described [57]. YPD medium, composed of 2% (w/v) peptone, 1% (w/v) yeast extract, and 2% (w/v) glucose, was used routinely for strain growth and maintenance. YPD medium containing 200 μg/ml of nourseothricin (Werner BioAgents, Jena, Germany) was used to select nourseothricin-resistant strains. Spider medium (pH 7.2), containing 1% (w/v) mannitol, 1% (w/v) nutrient broth, and 0.4% (w/v) dipotassium phosphate (Showa Chemical Industry, Japan), was used for opaque cell maintenance. All chemicals were purchased from Sigma-Aldrich Chemical, unless otherwise stated.
Growth curves were performed as described previously with slight modifications [58]. We measured the growth curves using a Biowave density meter (WPA CO8000). C. albicans strains were grown overnight in YPD, diluted to an optical density at 600 nm (OD600) of 0.1 in fresh YPD and incubated at 30°C and 100 rpm. The optical density was evaluated every 4 hr.
Plasmid and strain construction
C. albicans strains and oligonucleotides used in this study are listed in Tables S1 and S2, respectively. To generate each mutant strain, the 5′ flanking and 3′ flanking regions of each gene were PCR amplified using different primer sets and individually cloned into pSFS2A [59]. These constructs were linearized with restriction enzymes and transformed into C. albicans to generate each deletion mutant strain.
To generate the orf19.1539Δ strains, the 5′ flanking and 3′ flanking regions of ORF19.1539 were PCR amplified using primers 395/396 and 397/398, respectively. The 5′ and 3′ PCR products were digested with ApaI/XhoI and SacII/SacI, respectively, and cloned into the plasmid pSFS2A to generate the plasmid pSFS2A-orf19.1539 KO. The plasmid was digested with ApaI/SacI and transformed into the wild-type strain P37005 to generate heterozygous orf19.1539Δ/ORF19.1539 mutants. The SAT1 marker was recycled by treatment with 2% maltose. The heterozygous strains were retransformed with the same deletion construct to generate the orf19.1539Δ/orf19.1539Δ strains YL1031/YL1032.
To generate orf19.1725Δ strains, the 5′ flanking and 3′ flanking regions of ORF19.1725 were PCR amplified using primers 363/364 and 365/366, respectively. The 5′ and 3′ PCR products were digested with ApaI/XhoI and SacII/SacI, respectively, and cloned into the plasmid pSFS2A to generate the plasmid pSFS2A-orf19.1539 KO. The plasmid was digested with ApaI/SacI and transformed into P37005 to generate heterozygous orf19.1725Δ/ORF19.1725 mutants. The SAT1 marker was recycled, and the strains were retransformed with the same construct to generate orf19.1725Δ/orf19.1725Δ strains YL1033/YL1034. The ORF19.1725 complementation construct was made by amplification of its endogenous promoter and open reading frame (ORF) using primer 647/669. The PCR product was digested with ApaI/XhoI and cloned into pSFS-orf19.1725 KO to generate pSFS-orf19.1725AB. The plasmid was digested with ApaI/SacI and transformed into orf19.1725Δ to create YL1241/YL1242.
To generate orf19.2430Δ strains, the 5′ flanking and 3′ flanking regions of ORF19.2430 were PCR amplified using primers 323/324 and 325/326, respectively. The 5′ and 3′ PCR products were digested with ApaI/XhoI and SacII/SacI, respectively, and cloned into the plasmid pSFS2A to generate the plasmid pSFS2A-orf19.2430 KO. The plasmid was digested with ApaI/SacI and transformed into P37005 to generate heterozygous orf19.2430Δ/ORF19.2430 mutants. Homozygous orf19.2430Δ/orf19.2430Δ strains YL1037/YL1038 were created after the SAT1 marker was removed and the strains were retransformed with the same deletion construct.
To generate orf19.2691Δ strains, the 5′ flanking and 3′ flanking regions of ORF19.2691 were PCR amplified using primers 451/452 and 453/454, respectively. The 5′ and 3′ PCR products were digested with ApaI/XhoI and NotI/SacII, respectively, and cloned into the plasmid pSFS2A to generate the plasmid pSFS2A-orf19.2691 KO. The plasmid was digested with ApaI/SacI and transformed into MTLa/a to generate heterozygous orf19.2691Δ/ORF19.2691 mutants. The SAT1 marker was recycled and the strains retransformed with the same deletion construct to generate orf19.2691Δ/orf19.2691Δ strains YL1067/YL1068.
To generate orf19.5557Δ strains, the 5′ flanking and 3′ flanking regions of ORF19.5557 were PCR amplified using primers 331/332 and 333/334, respectively. The 5′ and 3′ PCR products were digested with ApaI/XhoI and SacII/SacI, respectively, and cloned into the plasmid pSFS2A to generate the plasmid pSFS2A-orf19.5557 KO. The plasmid was digested with ApaI/SacI and transformed into MTLa/a to generate heterozygous orf19.5557Δ/ORF19.5557 mutants. The SAT1 marker was recycled and the strains retransformed with the deletion construct to generate orf19.5557Δ/orf19.5557Δ strains YL1087/YL1088.
Opaque cells of each strain were obtained and purified after treatment with Lee's NAG (Table S1).
Hyphal formation tests
Overnight culture of the wild-type P37005, the orf19.1725 mutant strains and the complemented strains were grown in YPD supplemented with 0% serum or 50% serum for 12 hr. The hyphal development of each sample in different culture conditions was examined by the use of an Eclipse Ti inverted microscope (Nikon Instruments Inc., Melville, NY, USA).
Pheromone-stimulated cell adhesion assays
Approximately 5 × 107 cells of overnight cultures from Spider medium were added to 1 ml of Lee's medium with or without 0.01% dimethyl sulfoxide (DMSO) or 10 μM α-pheromone (GFRLTNFGYFEPG; synthesized by GMbiolab) in a 12-well plastic plate (Costar, Corning) [60]. The inoculated plates were incubated at room temperature for 16 hr without shaking. Each sample was washed with phosphate-buffered saline (PBS) to remove the non-adhered cells. The samples were then photographed, and the adhered cells were scratched out of the plastic well using a sterilized tip and quantified by measuring the OD600. The experiment was performed in three experimental replicates with each replicate containing two technical repeats
Conventional biofilm assay
Conventional biofilm assays were performed based on the previously established protocol to measure fungal mass in a silicone model [34]. Sterile silicone squares (Bentec Medical, PR72034-06N, 1.5 cm × 1.5 cm) were weighed before incubating in bovine serum (Sigma B-9433) in a 12-well plastic plate. The plastic plates were shaken at 150 rpm at 37°C overnight. Each serum-treated silicone square was washed with 2 ml PBS and placed in new sterilized 12-well culture dishes containing 2 ml Spider medium. Approximately 2 × 107 cells from YPD overnight cultures of the C. albicans P37005 wild-type and mutant strains were gently added on top of the silicone squares. Inoculated samples were incubated at 37°C and 150 rpm for 90 min for adhesion. Silicone squares were washed with 2 ml PBS and then incubated in 2 ml fresh Spider medium for 60 hr at 37°C and 150 rpm. The dry weight of the biofilm mass of each silicone square was determined after the supernatant was removed, and the silicone squares were dried overnight. The biofilm assays were performed in three experimental replicates with each replicate containing two technical repeats.
Quantitative mating assays
The opaque P37005 MTLa/a wild-type strain and mutant strains (SAT−, Arg+) and the MTLα/α DSY211 strain (SAT+, Arg−) [40] were grown in Spider medium at room temperature [57]. Approximately 2 × 107 MTLa/a and MTLα/α cells were mixed together on a nitrocellulose filter on Spider medium. Cells were incubated for 48 hr at 25°C and then resuspended in 1 ml sterilized ddH2O and plated onto selective media (SAT+, Arg−) to quantitate mating frequency, as previously described [34]. The experiment was done in three experimental replicates with each replicate containing two technical repeats.
Adhesion assays using zebrafish egg
Zebrafish embryos were generated from zebrafish mating as described previously [61]. Briefly, zebrafish embryos were collected after male and female zebrafishes mated. Embryos were then sterilized using 0.028% chlorine bleach containing 0.0017% sodium hypochlorite. The bleach solution was removed, and the embryos were washed twice in 40 ml sterilized ddH2O. The embryos were bleached again and washed with egg water (0.03% sea salt) 3 times. Twenty embryos were transferred into each well of a 24-well plate with Roswell Park Memorial Institute 1640 medium (RPMI). A total of 5 × 105 C. albicans cells of the wild-type P37005 strain and the orf19.1725Δ strains were co-incubated with embryos for 4 hr at 30°C. The embryos were then transferred into conical tubes containing 40 ml egg water and washed twice to remove non-adhered cells at 100 rpm for 3 min. The embryos were transferred into a new 24-well plate containing 0.7 ml egg water with 0.5% YPD. A Leica TCS SP5 II inverted microscope was used for confocal imaging. To quantitate adherent cells, embryos were sacrificed, disrupted by a FastPrep-24 instrument (MP Biomedicals, Illkirch, France), diluted and plated on YPD plates, and the number of CFUs was counted. The experiment was performed in three experimental replicates with each replicate containing two technical repeats. Statistical significance was determined using Student's t-test.
Virulence of C. albicans using zebrafish egg infection model
Twenty embryos were co-incubated with 5 × 105 C. albicans cells in 6-well plates containing 4 ml of RPMI serum at 30°C with shaking at 80 rpm for 4 hr for each experiment [61]. Non-adhered C. albicans cells were removed, and the embryos were incubated in egg water. To determine whether embryos were dead or alive, a Leica TCS SP5 II inverted microscope was used to confirm whether the infected embryo's heart was still beating. Survival rate was determined by calculating the number of infected zebrafish embryos that still had heartbeats divided by the total embryo number in each well after 24 hr and 48 hr of incubation at 30°C. Virulence assays were performed in three experimental replicates with each replicate containing two technical repeats. Statistical significance was determined using Student's t-test.
Statistical analyses
All statistical analyses were performed with Excel software. Differences between the wild-type and mutant strains were analyzed using a one-tailed, two-typed Student's t-test with a 95% confidence interval. All the experiments were performed in three experimental replicates with each replicate containing two technical repeats. The data are presented as the mean ± SD, and P < 0.05 was considered statistically significant.
Supplementary Material
Funding Statement
This work was supported by the Ministry of Science and Technology, Taiwan, MOST102-2320-B-002-027-MY3.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Hsiu-Jung Lo and members of her laboratory for technical support regarding the zebrafish embryo infection model. We thank Miss Ting-An Lin who was extremely helpful and provided invaluable assistance. This research was supported by MOST102-2320-B-002-027-MY3 from the Ministry of Science and Technology, R.O.C.
References
- [1].Neville BA, d'Enfert C, Bougnoux ME. Candida albicans commensalism in the gastrointestinal tract. FEMS Yeast Res. 2015;15(7):fov081. [DOI] [PubMed] [Google Scholar]
- [2].Papon N, Courdavault V, Clastre M, et al.. Emerging and emerged pathogenic Candida species: beyond the Candida albicans paradigm. PLoS Pathog. 2013;9(9):e1003550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cassone A, Cauda R. Candida and candidiasis in HIV-infected patients: where commensalism, opportunistic behavior and frank pathogenicity lose their borders. AIDS. 2012;26(12):1457–72. [DOI] [PubMed] [Google Scholar]
- [4].Noble SM, Gianetti BA, Witchley JN. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat Rev Microbiol. 2017;15(2):96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mallick EM, Bergeron AC, Jones SK Jr., et al.. Phenotypic plasticity regulates Candida albicans interactions and virulence in the vertebrate host. Front Microbiol. 2016;7:780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tao L, Du H, Guan G, et al.. Discovery of a “white-gray-opaque” tristable phenotypic switching system in Candida albicans: roles of non-genetic diversity in host adaptation. PLoS Biol. 2014;12(4):e1001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol. 2011;9(10):737–48. [DOI] [PubMed] [Google Scholar]
- [8].Slutsky B, Staebell M, Anderson J, et al. "White-opaque transition": a second high-frequency switching system in Candida albicans. J Bacteriol. 1987;169(1):189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pande K, Chen CB, Noble SM. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nat Genet. 2013;45(9):1088–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kvaal C, Lachke SA, Srikantha T, et al.. Misexpression of the opaque-phase-specific gene PEP1 (SAP1) in the white phase of Candida albicans confers increased virulence in a mouse model of cutaneous infection. Infect Immun. 1999;67(12):6652–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hull CM, Raisner RM, Johnson AD. Evidence for mating of the "asexual" yeast Candida albicans in a mammalian host. Science. 2000;289(5477):307–10. [DOI] [PubMed] [Google Scholar]
- [12].Magee BB, Magee PT. Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science. 2000;289(5477):310–3. [DOI] [PubMed] [Google Scholar]
- [13].White TC, Agabian N. Candida albicans secreted aspartyl proteinases: isoenzyme pattern is determined by cell type, and levels are determined by environmental factors. Journal of Bacteriology. 1995;177(18):5215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Geiger J, Wessels D, Lockhart SR, et al.. Release of a potent polymorphonuclear leukocyte chemoattractant is regulated by white-opaque switching in Candida albicans. Infect Immun. 2004;72(2):667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lohse MB, Johnson AD. Differential phagocytosis of white versus opaque Candida albicans by Drosophila and mouse phagocytes. PLoS One. 2008;3(1):e1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lewis LE, Bain JM, Lowes C, et al.. Stage specific assessment of Candida albicans phagocytosis by macrophages identifies cell wall composition and morphogenesis as key determinants. PLoS Pathog. 2012;8(3):e1002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sasse C, Hasenberg M, Weyler M, et al.. White-opaque switching of Candida albicans allows immune evasion in an environment-dependent fashion. Eukaryot Cell. 2013;12(1):50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gabrielli E, Sabbatini S, Roselletti E, et al.. In vivo induction of neutrophil chemotaxis by secretory aspartyl proteinases of Candida albicans. Virulence. 2016;7(7):819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zordan RE, Miller MG, Galgoczy DJ, et al.. Interlocking transcriptional feedback loops control white-opaque switching in Candida albicans. Plos Biology. 2007;5(10):2166–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tuch BB, Mitrovich QM, Homann OR, et al.. The transcriptomes of two heritable cell types illuminate the circuit governing their differentiation. PLoS Genet. 2010;6(8):e1001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ene IV, Lohse MB, Vladu AV, et al.. Phenotypic profiling reveals that Candida albicans opaque cells represent a metabolically specialized cell state compared to default white cells. Mbio. 2016;7(6):e01269–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Huang G, Yi S, Sahni N, et al.. N-acetylglucosamine induces white to opaque switching, a mating prerequisite in Candida albicans. PLoS Pathog. 2010;6(3):e1000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Huang G, Srikantha T, Sahni N, et al.. CO2 regulates white-to-opaque switching in Candida albicans. Current Biology. 2009;19(4):330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Alby K, Bennett RJ. Stress-induced phenotypic switching in Candida albicans. Mol Biol Cell. 2009;20(14):3178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sonneborn A, Tebarth B, Ernst JF. Control of white-opaque phenotypic switching in Candida albicans by the Efg1p morphogenetic regulator. Infect Immun. 1999;67(9):4655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lohse MB, Johnson AD. Identification and characterization of Wor4, a new transcriptional regulator of white-opaque switching. G3 (Bethesda). 2016;6(3):721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Huang G, Wang H, Chou S, et al.. Bistable expression of WOR1, a master regulator of white-opaque switching in Candida albicans. Proc Natl Acad Sci U S A. 2006;103(34):12813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hernday AD, Lohse MB, Nobile CJ, et al.. Ssn6 defines a new level of regulation of white-opaque switching in Candida albicans and is required for the stochasticity of the switch. MBio. 2016;7(1):e01565–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hernday AD, Lohse MB, Fordyce PM, et al.. Structure of the transcriptional network controlling white-opaque switching in Candida albicans. Mol Microbiol. 2013;90(1):22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Miller MG, Johnson AD. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell. 2002;110(3):293–302. [DOI] [PubMed] [Google Scholar]
- [31].Lin CH, Kabrawala S, Fox EP, et al.. Genetic control of conventional and pheromone-stimulated biofilm formation in Candida albicans. PLoS Pathog. 2013;9(4):e1003305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Daniels KJ, Srikantha T, Pujol C, et al.. Role of Tec1 in the development, architecture, and integrity of sexual biofilms of Candida albicans. Eukaryot Cell. 2015;14(3):228–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Daniels KJ, Srikantha T, Lockhart SR, et al.. Opaque cells signal white cells to form biofilms in Candida albicans. EMBO J. 2006;25(10):2240–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nobile CJ, Fox EP, Nett JE, et al.. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell. 2012;148(1-2):126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Finkel JS, Mitchell AP. Genetic control of Candida albicans biofilm development. Nat Rev Microbiol. 2011;9(2):109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Desai JV, Mitchell AP. Candida albicans biofilm development and its genetic control. Microbiol Spectr. 2015;3(3):MB-0005-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Singleton DR, Masuoka J, Hazen KC. Surface hydrophobicity changes of two Candida albicans serotype B mnn4Δ; mutants. Eukaryot Cell. 2005;4(4):639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lewis LE, Bain JM, Lowes C, et al.. Stage specific assessment of Candida albicans phagocytosis by macrophages identifies cell wall composition and morphogenesis as key determinants. PLoS Pathog. 2012;8(3):e1002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hall RA, Gow NA. Mannosylation in Candida albicans: role in cell wall function and immune recognition. Mol Microbiol. 2013;90(6):1147–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Alby K, Schaefer D, Bennett RJ. Homothallic and heterothallic mating in the opportunistic pathogen Candida albicans. Nature. 2009;460(7257):890–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nobile CJ, Mitchell AP. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p Curr Biol. 2005;15(12):1150–5. [DOI] [PubMed] [Google Scholar]
- [42].Richard ML, Plaine A. Comprehensive analysis of glycosylphosphatidylinositol-anchored proteins in Candida albicans. Eukaryot Cell. 2007;6(2):119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].de Groot PW, Bader O, de Boer AD, et al.. Adhesins in human fungal pathogens: glue with plenty of stick. Eukaryot Cell. 2013;12(4):470–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chaudhuri R, Ansari FA, Raghunandanan MV, et al.. FungalRV: adhesin prediction and immunoinformatics portal for human fungal pathogens. BMC Genomics. 2011;12:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Emanuelsson O, Brunak S, von Heijne G, et al.. Locating proteins in the cell using TargetP, SignalP and related tools. Nature Protocols. 2007;2(4):953–971. [DOI] [PubMed] [Google Scholar]
- [46].Pierleoni A, Martelli PL, Casadio R. PredGPI: a GPI-anchor predictor. BMC Bioinformatics. 2008;9:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Crooks GE, Hon G, Chandonia JM, et al.. WebLogo: a sequence logo generator. Genome Research. 2004;14(6):1188–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sundstrom P. Adhesion in Candida spp. Cell Microbiol. 2002;4(8):461–9. [DOI] [PubMed] [Google Scholar]
- [49].Nobile CJ, Nett JE, Andes DR, et al.. Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot Cell. 2006;5(10):1604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Boisrame A, Cornu A, Da Costa G, et al.. Unexpected role for a serine/threonine-rich domain in the Candida albicans Iff protein family. Eukaryot Cell. 2011;10(10):1317–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zeitlinger J, Simon I, Harbison CT, et al.. Program-specific distribution of a transcription factor dependent on partner transcription factor and MAPK signaling. Cell. 2003;113(3):395–404. [DOI] [PubMed] [Google Scholar]
- [52].Chou S, Huang L, Liu H. Fus3-regulated Tec1 degradation through SCFCdc4 determines MAPK signaling specificity during mating in yeast. Cell. 2004;119(7):981–90. [DOI] [PubMed] [Google Scholar]
- [53].Taschdjian CL, Burchall JJ, Kozinn PJ. Rapid identification of Candida albicans by filamentation on serum and serum substitutes. AMA J Dis Child. 1960;99:212–5. [DOI] [PubMed] [Google Scholar]
- [54].Mardon D, Balish E, Phillips AW. Control of dimorphism in a biochemical variant of Candida albicans. J Bacteriol. 1969;100(2):701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Buffo J, Herman MA, Soll DR. A characterization of pH-regulated dimorphism in Candida albicans. Mycopathologia. 1984;85(1–2):21–30. [DOI] [PubMed] [Google Scholar]
- [56].Si H, Hernday AD, Hirakawa MP, et al.. Candida albicans white and opaque cells undergo distinct programs of filamentous growth. PLoS Pathog. 2013;9(3):e1003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Liang SH, Cheng JH, Deng FS, et al.. A novel function for Hog1 stress-activated protein kinase in controlling white-opaque switching and mating in Candida albicans. Eukaryot Cell. 2014;13(12):1557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Thewes S, Moran GP, Magee BB, et al.. Phenotypic screening, transcriptional profiling, and comparative genomic analysis of an invasive and non-invasive strain of Candida albicans. BMC Microbiology. 2008;8:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Reuss O, Vik A, Kolter R, et al.. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene. 2004;341:119–27. [DOI] [PubMed] [Google Scholar]
- [60].Chang WH, Liang SH, Deng FS, et al.. The conserved dual phosphorylation sites of the Candida albicans Hog1 protein are crucial for white-opaque switching, mating, and pheromone-stimulated cell adhesion. Med Mycol. 2016;54(6):628–40. [DOI] [PubMed] [Google Scholar]
- [61].Chen YZ, Yang YL, Chu WL, et al.. Zebrafish egg infection model for studying Candida albicans adhesion factors. PLoS One. 2015;10(11):e0143048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


