Abstract
The importance of multidrug resistance-associated protein 4 (Mrp4/Abcc4) in limiting the penetration of Mrp4 substrate compounds into the central nervous system across the blood–brain barrier (BBB) was investigated using Mrp4-/- mice. Significant ATP-dependent uptake by MRP4 was observed for ochratoxin A, pitavastatin, raltitrexed (Km 43.7 μM), pravastatin, cGMP, 2,4-dichlorophenoxyacetate, and urate. The defect in the Mrp4 gene did not affect the brain-to-plasma ratio (Kp,brain) of quinidine and dantrolene. Following intravenous infusion in wild-type and Mrp4-/- mice, the plasma concentrations of the tested compounds (cefazolin, cefmetazole, ciprofloxacin, cyclophosphamide, furosemide, hydrochlorothiazide, methotrexate, pitavastatin, pravastatin, and raltitrexed) were identical; however, Mrp4-/- mice showed a significantly higher (1.9–2.5-fold) Kp,brain than wild-type mice for methotrexate, raltitrexed, and cyclophosphamide. GF120918, a dual inhibitor of P-gp and Bcrp, significantly decreased the Kp,cortex and Kp,cerebellum only in Mrp4-/- mice. Methotrexate and raltitrexed are also substrates of multispecific organic anion transporters such as Oatp1a4 and Oat3. GF120918 showed an inhibition potency against Oatp1a4, but not against Oat3. These results suggest that Mrp4 limits the penetration of methotrexate and raltitrexed into the brain across the BBB, which is likely to be facilitated by some uptake transporters.
Keywords: Blood–brain barrier, MRP, P-glycoprotein, cerebrospinal fluid, ABC transporters
Introduction
The delivery of drugs to the central nervous system (CNS) is critical to exert their effect on the CNS. It is well known that the blood–brain barrier (BBB) limits the penetration of drugs into the CNS from the blood circulation system, thereby lowering their pharmacological effect. Because of the highly developed tight junctions between the adjacent cells, the brain capillary endothelial cells act as a diffusive barrier.1,2 In addition, adenosine triphosphate (ATP)-binding cassette (ABC) transporters, such as P-glycoprotein (P-gp/MDR1/ABCB1) and breast cancer resistance protein (BCRP/ABCG2) act as active barrier in the endothelial cells by mediating the active efflux across the luminal membrane into the blood circulation. 2,3,4 Animal studies using the inhibitors or genetic modification have demonstrated that a lack of P-gp greatly enhanced the brain-to-plasma concentration ratios (Kp,brain) of various drugs, supporting the key role of P-gp in the BBB.3 In addition, a defect of BCRP resulted in a significant increase in the Kp,brain of neutral and weak acids, such as dantrolene and phytoestrogens, whereas those of some substrates were only marginally changed.5,6 Because of an overlapping substrate specificity between P-gp and BCRP, the double knockout of P-gp and Bcrp showed higher Kp,brain than the knockout of either P-go or Bcrp, indicating that some common substrate drugs undergo active efflux by both P-gp and Bcrp.7
MRP4 is also an ABC transporter expressed in many tissues involving the kidney and brain.8 In the brain, MRP4 is mainly expressed in the blood-facing membrane of the brain capillary and choroid plexus.4 Its membrane localization indicates that MRP4 mediates the luminal efflux of its substrate drugs. In fact, Mrp4 limits the penetration of topotecan4 and adefovir9 into the brain at the BBB, although we could not observe a significant change for adefovir, cidofovir, and tenofovir.10 We demonstrated that the elimination of Ro 64-0802, the active form of oseltamivir, from the brain after injection into the cerebral cortex, was significantly delayed in Mrp4-/- mice.11 Furthermore, after subcutaneous administration of Ro 64-0802 using an osmotic pump, the Kp,brain was significantly higher in Mrp4-/- mice than in wild-type (WT) mice. On other hand, organic anion transporter 3 (Oat3)-/- mice showed a delayed elimination of Ro 64-0802 from the brain due to reduced uptake across the abluminal membrane. Nevertheless, the Kp,brain was unchanged in Oat3-/- mice following intravenous injection.11 Thus, we speculated that Mrp4 can facilitate the elimination of its substrates from the CNS, and can also limit the entry from the blood circulation at the BBB. Many anionic drugs have been found to be MRP4 substrates in vitro,12,13,14,15,16,17,18 and in vivo studies using Mrp4-/- mice19 have revealed that Mrp4 mediates the urinary excretion of some compounds. Generally, the distribution volume of organic anions in the brain is quite low because of their poor lipid membrane permeability. A multispecific organic anion transporter organic anion-transporting polypeptide 1a4 (Oatp1a4) is expressed in the abluminal membrane to mediate the uptake of organic anions; however, the Kp,brain values of pitavastatin and taurocholate were unchanged between WT and Oatp1a4-/- mice, the absolute values of which were close to the capillary volume.20 Thus, we speculated that such a limited distribution of organic anions is explained by the active efflux by Mrp4 at the BBB.
In the present study, we compared the Kp,brain of drugs between WT and Mrp4-/- mice to identify the Mrp4 substrates for which the Kp,brain is determined by Mrp4 at the BBB following a long exposure using an osmotic pump implanted under the skin of mice.
Materials & Methods
Materials
[3H]Dehydroepiandrosterone sulfate ([3H]DHEAS, 60.0 Ci/mmol), [3H]taurocholate (5.0 Ci/mmol), and [3H]estrone sulfate (45 Ci/mmol) were purchased from PerkinElmer Life Science, (Boston, MA). [3H]2,4-D (20.0 Ci/mmol) was purchased from American Radiolabeled Chemicals (St. Louis, MO). [3H]Methotrexate (12.6 Ci/mmol), [3H]OchratoxinA (21.3 Ci/mmol), [14C]urate (53 mCi/mmol), and [3H]cyclic guanosine monophosphate ([3H]cGMP, 2.4 Ci/mmol) were purchased from Moravek Biochemicals, Inc (Brea, CA). [3H]pravastatin (45.5 Ci/mmol) and unlabeled pravastatin were kindly gifted by Daiichi Sankyo. Co., Ltd (Tokyo, Japan). [Fluorobenzene-U-14C]pitavastatin (11.7 mCi/mmol) was synthesized by Amersham Biosciences UK, Ltd. (Little Chalfont, Buckinghamshire, UK). Raltitrexed was donated by Hospira UK Limited (Warwickshire, UK). GF120918 (Elacridar) was donated from GlaxoSmithKline (Ware, UK). Methotrexate was purchased from Wako Pure Chemicals (Osaka, Japan), and ATP, creatine phosphate, creatine phosphokinase, cefazolin, cefmetazole, furosemide, and hydrochlorothiazide were purchased from Sigma-Aldrich (St. Louis, MO). Dantrolene (Dantrium), ciprofloxacin, and pitavastatin were purchased from LKT Laboratories Inc. (St. Paul, MN). Quinidine was purchased from Tokyo Kasei (Tokyo, Japan). Cyclophosphamide was purchased from MP biomedicals LLC (Solon, OH). All other chemicals were commercially available and of reagent grade.
Animals
Mrp4-/- mice had been established previously.4 Male C57BL/6J, and Mrp4-/- mice were maintained by CLEA Japan, Inc. (Tokyo, Japan). All mice (11–31 weeks old) were maintained at a controlled temperature under a 12-h light/dark cycle. Food and water were made available ad libitum. All experiments using animals in the present study were performed according to the guidelines provided by the Institutional Animal Care Committee (Graduate School of Pharmaceutical Sciences, University of Tokyo).
In vitro Transport Studies with Membrane Vesicles
Membrane vesicles were prepared from HEK293 cells infected with recombinant adenovirus harboring hMRP4 and green fluorescent protein gene as described previously.10,19 The transport studies were performed using a rapid filtration technique. In brief, 15 μL of transport medium (10 mM Tris-HCl, 250 mM sucrose, and 10 mM MgCl2 at pH 7.4) containing substrates was preincubated at 37°C for 3 min and then rapidly and gently mixed with 5 μL of membrane vesicle suspension (5–10 μg of protein). The reaction mixture contained 5 mM ATP or adenosine monophosphate (AMP) along with the ATP-regenerating system (10 mM creatine phosphate and 100 μg/μL creatine phosphokinase). After incubation at 37°C for 5-min, the transport reaction was terminated by adding of 1-mL of ice-cold buffer containing 10 mM Tris-HCl, 250 mM sucrose, and 0.1 M NaCl at pH 7.4. The halted reaction mixture was passed through a 0.45 μm HA filter (Millipore Corp., Bedford, MA), and then washed twice with 5 mL of stop solution. The radioactivity retained on the filter was measured in a liquid scintillation counter (LS 6000SE, Beckman Instruments, Fullerton, CA) after the addition of scintillation cocktail (Clear-sol I, Nacalai Tesque, Tokyo, Japan). For non-radiolabeled compounds, a 0.45-μm MF™-membrane filter (HAWP; Millipore Corp.) was used for filtration. After twice wash, substrates retained on the filter were recovered in 1 mL methanol containing an internal standard by sonication for 15 min. After centrifugation, the supernatants were evaporated using a centrifugal concentrator (CC-105; TOMY, Tokyo, Japan) and dissolved in 50 μL 0.025% HCOOH/50% Methanol. Subsequently, 20-μl aliquots were used for liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis quantification as described below. To determine concentration dependency, the initial uptake rates of raltitrexed by membrane vesicles at 37°C for 5 min was measured at different concentrations (3–1000 μM). ATP-dependent uptake was obtained by subtracting transport velocity in the presence of AMP from that in the presence of ATP. Km and Vmax values were calculated using the Eadie–Hofstee plots.
Tissue-to-Plasma Concentration Ratio after Intravenous Infusion of Compounds in WT and Mrp4-/-Mice
Male C57BL/6J and Mrp4-/- mice (13–31 weeks old), weighing approximately 24–38 g, were used for these experiments. Under pentobarbital anesthesia (50 mg/kg), the jugular vein was cannulated with a polyethylene-10 catheter for the injection of test compounds.
Infusion study of quinidine and dantrolene
The infusion rates of quinidine and dantrolene (dissolved in a 2:3 mixture of propylene glycol/saline) were 8 and 2 μmol/h/kg after priming doses of 6 and 4 μmol/kg, respectively. Blood samples were collected from the jugular vein at 60, 90, and 120 min. The mice were sacrificed after 120 min and the cerebrospinal fluid (CSF) and brain were excised immediately.
Infusion study of methotrexate and raltitrexed
The infusion rates of methotrexate and raltitrexed were 8 and 12 μmol/h/kg, respectively, after priming doses of 2 μmol/kg for both. GF120918 [17 μmol (10 mg) / 3.3 mL/kg, dissolved in a 3:2 mixture of polyethylene glycol:water]21 was intravenously injected into mice 15 min before the intravenous infusion of methotrexate and raltitrexed (cassette dosing). Blood samples were collected from the jugular vein at 60, 75, and 90 min. The mice were sacrificed after 90 min, and the cortex, cerebellum, and kidney were excised immediately.
Plasma was obtained by centrifugation of the blood samples (at 4 °C, 15,000 × g, for 10 min). The plasma, CSF, and tissue concentrations of compounds were determined by LC-MS/MS analysis. Tissue-to-plasma concentration ratios (Ctissue/Cplasma, Kp,tissue) were obtained by dividing the compound concentrations in the tissue by the plasma concentrations measured at the last sampling point.
Brain-to-Plasma and CSF-to-Plasma Concentration Ratio after Subcutaneous Infusion of Compounds in WT and Mrp4-/- Mice
Male or female C57BL/6J and Mrp4-/- mice (11–22 weeks old) weighing approximately 20–35 g were used for these experiments. An osmotic pump (8 μL/h; Alzet, Cupertino, CA) was implanted under the skin in the backs of the mice under pentobarbital anesthesia (50 mg/kg). The mice received a continuous subcutaneous infusion of compounds at doses ranging from 32 to 400 μg/h/head. Blood samples were collected from the postcaval vein at 18–24 h after treatment under pentobarbital anesthesia, and the brain and CSF were excised immediately. Plasma was obtained by centrifugation of the blood samples (at 4 °C, 15,000 × g, for 10 min). The plasma, brain, and CSF concentrations of compounds were determined by LC-MS /MS analysis.
Transport Study with Mock- and mOatp1a4-Expressing HEK293 Cells and Mock- and mOat3-Expressing Flp-in293 Cells
mOatp1a4-expressing HEK293 cells 20 and mOat3-expressing Flp-in293 cells were constructed as reported previously.22 Mock- and mOatp1a4-expressing HEK293 cells were grown in Dulbecco’s modified Eagle’s medium low glucose (Gibco, Thermo Fisher Scientific Inc., Waltham, MA) supplemented with 10% fetal bovine serum (American Type Culture Collection, Manassas, VA) and Mock- and mOat3-expressing Flp-in293 cells were grown in Dulbecco’s modified Eagle’s medium high glucose supplemented with 10% fetal bovine serum at 37°C with 5% CO2 and 95% humidity. Cells were then seeded in 12-well plates coated with poly-L-lysine/poly-L-ornithine at a density of 1.5–2.0 × 105 cells per well. After 2 d, the cell culture medium was replaced with culture medium supplemented with 5 mM sodium butyrate 24 h before the transport assay to induce the expression of exogenous transporters. Uptake was initiated by the addition of a Krebs-Henseleit buffer containing radiolabeled and/or unlabeled compounds after cells had been washed twice and preincubated with Krebs-Henseleit buffer in the presence or absence of inhibitor (for mOatp1a4; 0.01 to 1 μM GF120918, for mOat3; 100 μM probenecid and 0.1 to 1 μM GF120918) at 37°C for 15 min. The Krebs-Henseleit buffer consisted of 118 mM NaCl, 23.8 mM NaHCO3, 4.8 mM KCl, 1 mM KH2PO4, 1.2 mM MgSO4, 12.5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 5 mM glucose, and 1.5 mM CaCl2 adjusted to pH 7.4. After 2- (mOatp1a4) or 5-min (mOat3) incubation, the uptake was terminated by addition of ice-cold Krebs-Henseleit buffer, and cells were washed three times. For radiolabeled compounds, cells were then dissolved in 300 μL 1 N NaOH, neutralized with 300 μL 1 N HCl, and aliquots (400 μL) were transferred to scintillation vials. The radioactivity associated with the cells and incubation buffer was measured in a liquid scintillation counter (LSC-6101, Hitachi Aloka Medical, Ltd, Tokyo, Japan) after adding 5 mL of liquid scintillation cocktail (Insta-Gel Plus, PerkinElmer) to the scintillation vials. For unlabeled compounds, cells were homogenized in 300 μL 0.1 M phosphate-buffered saline with an ultrasonic probe. Subsequently, 100-μL aliquots were used for LC-MS/MS analysis quantification as described below. Aliquots of cell lysate and homogenate were used to determine the protein concentration by Bradford ULTRA (Expedeon, Inc, San Diego, CA) with bovine serum albumin as a standard. Compound uptake was calculated by the amount of compound associated with the cell specimens divided by the medium concentration.
Quantification using LC-MS/MS
Tissue was homogenized with a four-fold volume of phosphate-buffered saline to obtain a 20% tissue homogenate. Plasma, CSF, and 20% tissue homogenate (for cyclophosphamide analysis, after 2-h incubation with 0.2 M semicarbazide) were precipitated with two volumes of acetonitrile that contained an internal standard and centrifuged at 4°C and 15,000 × g for 10 min. The supernatants were diluted with water or evaporated if necessary, reconstituted in the mobile phase, and subjected to LC-MS/MS analysis. API5500 Qtrap or API4000 instrument (Applied Biosystems, Foster City, CA) equipped with a Shimadzu prominence series LC system (Shimadzu, Kyoto, Japan) (curtain gas; 30, collision gas; 7, ion spray voltage; 5,500, temperature; 500°C, ion source gas 1; 70 and ion source gas 2; 80) and a Micromass Quattromicro instrument equipped with an Alliance 2695 system (Waters) (capillary voltage; 4.5 kV, cone voltage; 22 V, source temperature; 120°C, and desolution temperature; 350°C) were used. All compounds were analyzed in the multireaction monitoring mode under electron spray ionization conditions. The analytical columns used were Xbridge C18 (2.5 um, 2.1 mmID × 50 mm; Waters, Milford, MA), Ascentis Express C18 (2.7 μm, 2.1 mm ID × 50 mm; Supelco, St. Louis, MO), or CAPCELL PAK C18 MGII (3 μm, 2 mm × 50 mm; Shiseido, Tokyo, Japan). The column temperature was 40°C. Detailed LC conditions and mass-to-charge ratios are shown in Supplementary Table 1.
Statistical Analysis
Data are presented as means ± standard error (S.E.) of three to four animals, unless otherwise specified. A Student’s two-tailed unpaired t test was used to identify significant differences between groups when appropriate. Statistical significance was set at P ≤ 0.05.
Results
ATP-Dependent Uptake of Anionic Compounds in Human MRP4-Expressing Membrane Vesicles
Consistent with a previous report,19 ATP markedly stimulated the uptake of DHEAS by MRP4-expressing membrane vesicles; the uptake values of DHEAS for 5 min at 37°C in the presence of AMP or ATP were 143 ± 3 and 1,443 ± 52 μL/mg protein, respectively (Table 1).
Table 1.
Transport properties by MRP4
| MRP4-expressing vesicle uptake
|
Reference | |||
|---|---|---|---|---|
| MRP4-ATP | MRP4-AMP | ATP/AMP ratio | ||
| μL/5min/mg | ||||
| cefazolina | 76.7 | 0.2 | 471 | 18) |
| cefmetazolea | 103 | 3 | 31 | 18) |
| methotrexate | 30.2 | 1.6 | 19 | |
| raltitrexed | 61.0 | 3.4 | 18 | |
| [3H]pravastatin | 18.4 | 1.7 | 11 | |
| [3H]DHEAS | 1443 | 143 | 10 | |
| [3H]pitavastatin | 162 | 43 | 3.8 | |
| furosemidea | 118 | 41 | 2.9 | 19) |
| hydrochlorothiazidea | 2.87 | 1.53 | 1.9 | 19) |
the transport activities were cited from the reference indicated for comparison.
ATP-dependent uptake mediated by MRP4 was observed for other compounds; ochratoxin A, pitavastatin, raltitrexed, pravastatin, cGMP, 2,4-D, and urate (Fig. 1). No or a low effect was observed in the membrane vesicles prepared from the host HEK293 cells (Fig. 1). We have previously reported the ATP-dependent uptake of methotrexate by MRP414. To confirm this, we demonstrated the ATP-dependent uptake of methotrexate by MRP4; the values were shown to be 30.2 (30.5 and 29.8) and 1.62 (1.85 and 1.39) μL/mg protein (duplicate determination) after a 5-min incubation in the presence of ATP and AMP, respectively, in MRP4-expressing membrane vesicles. These values were similar to the previously reported values. The uptake of raltitrexed by MRP4 was saturable with Km and Vmax values of 43.7 ± 6.9 μM and 614 ± 58 pmol/min/mg protein, respectively (Fig. 2).
Fig. 1. The uptake of various compounds by multidrug resistance-associated protein 4 (MRP4)-expressing membrane vesicles.
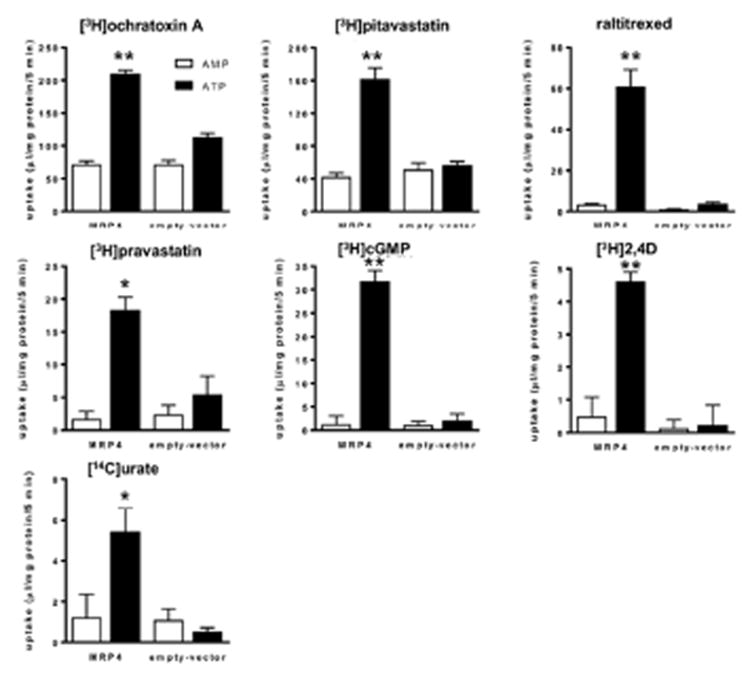
The uptake of [3H]ochratoxin A (0.1 μM), [3H]pitavastatin (0.1 μM), raltitrexed (30 μM), [3H]pravastatin (0.1 μM), [3H]cyclic guanosine monophosphate (cGMP, 0.1 μM), [3H]2,4D (0.1 μM), and [14C]urate (0.1 μM) by the membrane vesicles that were prepared from HEK293 cells expressing MRP4 and green fluorescent protein was determined at 37°C for 5 min in medium that contained 5 mM ATP (■) or AMP (□). Each bar with an error bar represents the mean value and standard error (S.E.) (n = 3).
Fig. 2. Transport properties of raltitrexed by multidrug resistance-associated protein 4 (MRP4).
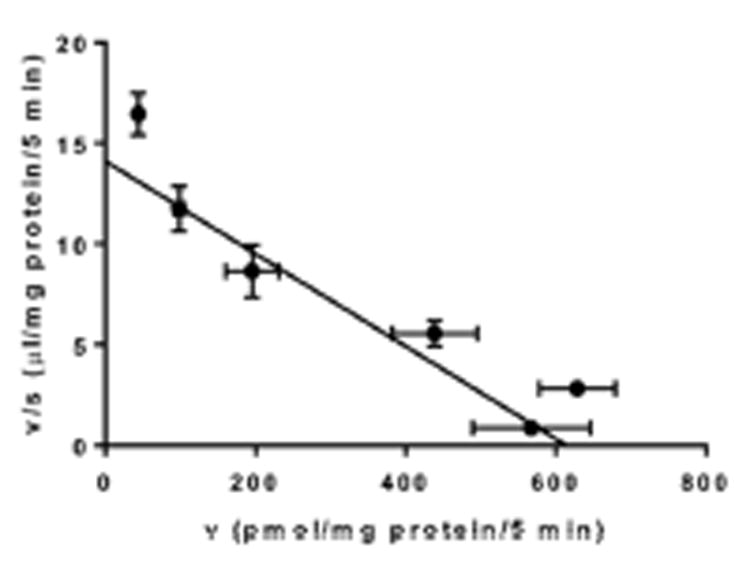
Concentration dependance of raltitrexed uptake is shown as Eadie-Hofstee plots. The solid line represents the fitted line obtained by nonlinear regression analysis. Each data point with an error bar represents the mean value and S.E. (n = 3).
Brain-to-Plasma and CSF-to-Plasma Concentration Ratio of Quinidine and Dantrolene after Intravenous Infusion of Quinidine and Dantrolene in WT and Mrp4-/- Mice
To confirm that the activities of P-gp and Bcrp are unchanged in Mrp4-/- mice, Kp,brain and the CSF-to-plasma concentration ratio (Kp,CSF) of quinidine and dantrolene were determined in WT and Mrp4-/- mice. The penetration of quinidine and dantrolene into the brain is selectively limited by P-gp and Bcrp, respectively.7 Quinidine or dantrolene were administered to WT and Mrp4-/- mice by intravenous infusion for 120 min, and the Kp,brain and Kp,CSF of quinidine and dantrolene was determined (Fig. 3). Neither Kp,brain nor Kp,CSF of quinidine and dantrolene was changed between WT and Mrp4-/- mice.
Fig. 3. Comparison of the plasma concentrations, brain-to-plasma concentration ratio, and cerebrospinal fluid (CSF)-to-plasma concentration ratio of quinidine (A) and dantrolene (B) after intravenous infusion in wild-type (WT) and Mrp4 -/- mice.
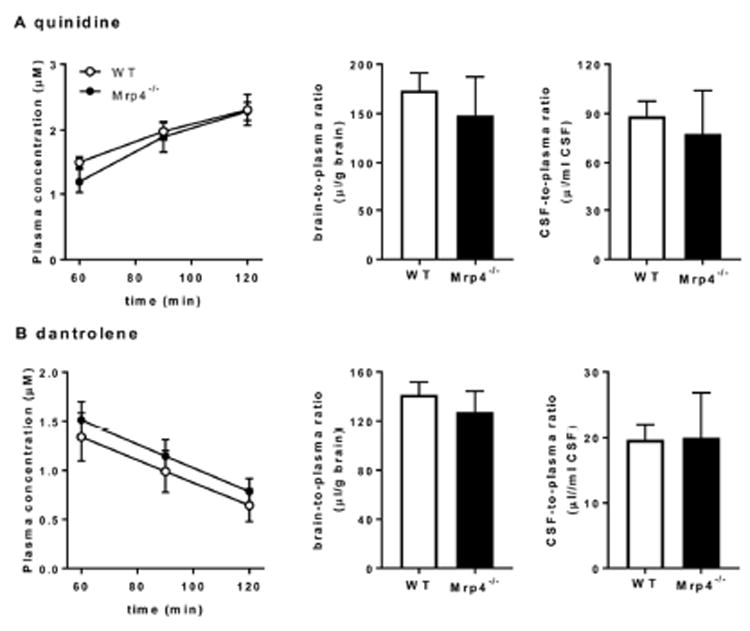
Mice received a continuous intravenous infusion for 120 min of either quinidine or dantrolene at a dose of 8 or 2 μmol/h/kg after priming doses of 6 or 4 μmol/kg, respectively. The plasma, brain, and CSF concentrations of quinidine and dantrolene were determined at the end of infusion. ■, data for WT mice; □, data for Mrp4-/- mice. Each data point and bar with an error bar represents the mean value and S.E. obtained from three to four mice.
Brain-to-plasma Concentration Ratio of Drugs after Subcutaneous Infusion in WT and Mrp4-/- Mice
To clarify the importance of the Mrp4-mediated efflux of methotrexate and raltitrexed at the BBB, methotrexate or raltitrexed were administered to mice by subcutaneous infusion for 19 h, and their Kp,brain and Kp,CSF were determined. The concentrations of methotrexate in the plasma were 1.5 ± 0.1 and 1.5 ± 0.1 μg/mL in WT and Mrp4-/- mice, respectively. The Kp,brain and Kp,CSF of methotrexate were 1.9-fold and 1.5-fold (without statistical significance, p = 0.074) higher in Mrp4-/- mice than that in WT mice, respectively. The concentrations of raltitrexed in the plasma were 3.6 ± 0.4 and 4.2 ± 0.5 μg/mL in WT and Mrp4-/- mice, respectively. The Kp,brain and Kp,CSF of raltitrexed were 2.5-fold and 1.8-fold higher in Mrp4-/- mice than that in WT mice, respectively (Fig. 4). Other drugs listed in Table 2 were administered by subcutaneous infusion for 19 h, and plasma and brain concentrations were determined in WT and Mrp4-/- mice. The plasma concentrations were similar between WT and Mrp4-/- mice. Among the tested drugs, the Kp,brain of cyclophosphamide was significantly higher in Mrp4-/- mice.
Fig. 4. Comparison of the plasma concentration, brain-to-plasma concentration ratio, and cerebrospinal fluid (CSF)-to-plasma concentration ratio of methotrexate (A) and raltitrexed (B) after subcutaneous infusion in wild-type (WT) and Mrp4-/- mice.
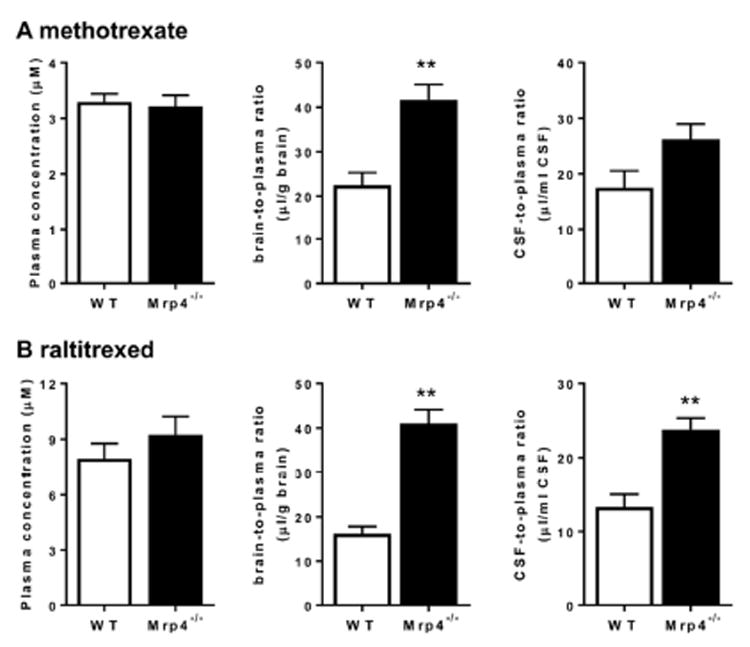
Mice received a continuous subcutaneous infusion of either methotrexate or raltitrexed at a dose of 80 or 160 μg/h/mouse, respectively, for 19 h with an osmotic pump. The plasma, brain and CSF concentrations of methotrexate and raltitrexed were determined at the end of the infusion. □, data for WT mice; ■, data for Mrp4-/- mice. Each bar with an error bar represents the mean value and S.E. obtained from four mice. Asterisks represent statistically significant differences between WT and Mrp4-/- mice: **, P < 0.01.
Table 2.
Comparison among plasma concentration and Kp ratios in wild-type and Mrp4-/- mice received a continuous subcutaneous infusion
| Dose (nmol/h) | Animals | n | Cp (μM) | Kp,brain | fold difference (KO/WT) | Kp,CSF | fold difference (KO/WT) | |
|---|---|---|---|---|---|---|---|---|
| cefazolin | 700 | WT | 4 | 35 ± 4 | 0.034 ± 0.009 | <1.0 | NT | |
| KO | 4 | 29 ± 0 | 0.015 ± 0.001 | |||||
| cefmetazole | 680 | WT | 4 | 8.7 ± 1.7 | 0.016 ± 0.012 | <1.0 | NT | |
| KO | 4 | 8.7 ± 0.8 | 0.008 ± 0.002 | |||||
| ciprofloxacin | 97 | WT | 4 | 0.18 ± 0.04 | 0.061 ± 0.016 | 1.6 | NT | |
| KO | 4 | 0.14 ± 0.02 | 0.096 ± 0.019 | |||||
| cyclophosphamide | 1500 | WT | 3 | 0.0073 ± 0.0015 | 0.73 ± 0.12 | 2.1* | NT | |
| KO | 3 | 0.0046 ± 0.0004 | 1.5 ± 0.1 | |||||
| furosemide | 120 | WT | 4 | 7.6 ± 1.5 | 0.010 ± 0.002 | 1.0 | NT | |
| KO | 4 | 7.9 ± 0.9 | 0.011 ± 0.001 | |||||
| hydrochlorothiazide | 1100 | WT | 4 | 10 ± 1 | 0.11 ± 0.01 | 1.2 | NT | |
| KO | 4 | 14 ± 3 | 0.13 ± 0.03 | |||||
| methotrexate | 180 | WT | 4 | 3.3 ± 0.2 | 0.022 ± 0.003 | 1.9 ** | 0.018±0.003 | 1.5 |
| KO | 4 | 3.3 ± 0.2 | 0.042 ± 0.003 | 0.026±0.003 | ||||
| pitavastatin | 95 | WT | 4 | 0.93 ± 0.10 | 0.031 ± 0.007 | <1.0 | NT | |
| KO | 4 | 1.0 ± 0.3 | 0.027 ± 0.010 | |||||
| pravastatin | 720 | WT | 4 | 1.4 ± 0.1 | 0.036 ± 0.003 | 1.2 | NT | |
| KO | 4 | 2.0 ± 0.3 | 0.042 ± 0.018 | |||||
| raltitrexed | 350 | WT | 4 | 7.9 ± 0.9 | 0.016 ± 0.002 | 2.5 ** | 0.013±0.002 | 1.8** |
| KO | 4 | 9.2 ± 1.1 | 0.041 ± 0.003 | 0.024±0.002 |
P < 0.05 and
P < 0.01
WT, wild-type mice; KO, Mrp4-/- mice; NT, not tested
Comparing the Brain-to-plasma Concentration Ratio of Mrp4 Substrate Drugs between WT and Mrp4-/- Mice
Methotrexate and raltitrexed were administered to WT and Mrp4-/- mice by intravenous infusion for 90 min following which their concentrations in the cerebrum, cerebellum, and kidney concentrations were measured to determine the tissue-to-plasma concentration ratio. There was no significant difference in the plasma concentrations of methotrexate and raltitrexed between WT and Mrp4-/- mice, whereas the cortex- or cerebellum-to-plasma ratio of methotrexate and raltitrexed were significantly higher in Mrp4-/- mice than in WT mice (Fig. 5). There was no regional difference in the effect of defect in the Mrp4 gene between the cortex and cerebellum. Pretreatment with GF120918 (17 μmol/kg, intravenous administration), a dual inhibitor for P-gp and Bcrp,23 blunted the effect of Mrp4 knockout. The kidney-to-plasma concentration ratio (Kp,kidney) of methotrexate and raltitrexed were similar between WT and Mrp4-/- mice, whereas administration of GF120918 significantly increased the ratio (Fig. 5).
Fig. 5. Effect of GF120918 on the cortex-, cerebellum-, and kidney-to-plasma ratios of methotrexate (A) and raltitrexed (B) in wild-type (WT) and Mrp4-/- mice.
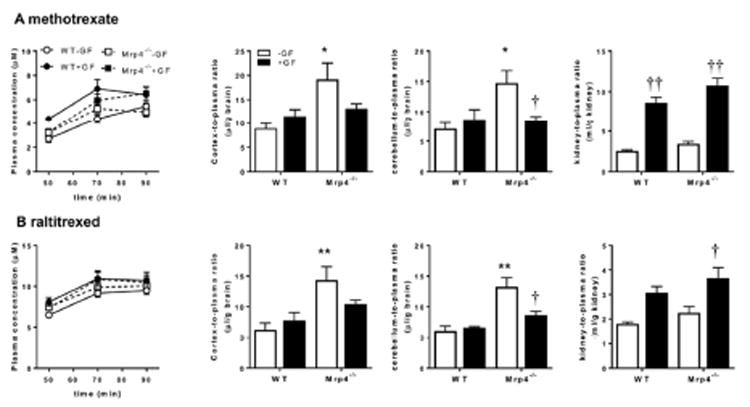
At 15 min after the injection of GF120918 (17 μmol/kg, closed symbols) or solvent alone (open symbols), the mice received a constant intravenous infusion of methotrexate and raltitrexed at doses of 8 and 12 μmol/h/kg. The plasma concentrations of methotrexate and raltitrexed were determined at the designated times in WT (circles) and Mrp4-/- mice (squares). The tissue-to-plasma ratio was calculated at 90 min. Each data point and bar with an error bar represent the mean value and standard error (S.E.) obtained from three to four mice. Asterisks represent statistically significant differences between WT and Mrp4-/- mice; *, P < 0.05; **, P < 0.005. Daggers represent statistically significant differences between control and GF120918-pretreated mice; †, P < 0.05; ††, P < 0.005; †††, P < 0.0005.
Effect of GF120918 on the Transport Activities of Oatp1a4 and Oat3
Beside the typical Oatp1a4 substrate, [3H]taurocholate, uptake of methotrexate and raltitrexed was significantly higher in Oatp1a4-expressing cells, which was inhibited by GF120918. For comparison, the activities of Oat3 were also tested. Overexpression of Oat3 in HEK293 cells also increased the cellular uptake of methotrexate and raltitrexed as well as the prototypical substrate, [3H]estrone sulfate. The uptake was inhibited by probenecid, but only weakly by GF120918.
Discussion
Understanding the drug transport systems in the BBB is a critical issue in drug development, as this understanding is required to optimize the permeability of drugs for their CNS effect. In the present study, we conducted an in vivo study using Mrp4-/- mice to expand the role of Mrp4 in the BBB.
In vitro transport studies identified some compounds as MRP4 substrates. The activities of ochratoxin A and pitavastatin were highest, followed by raltitrexed, pravastatin, cGMP, methotrexate, and to lesser degree, 2,4D and urate (Fig. 1). The Km value of raltitrexed was similar to that of its analog, methotrexate, which we reported previously (103 μM).14
Previously, we reported similar messenger RNA (mRNA) expression of multi-drug resistance protein 1a (Mdr1a) and Bcrp in the cerebral cortex between WT and Mrp4-/- mice.11 Consistent with this result, the Kp,brain and Kp,CSF of quinidine and dantrolene, reference compounds of the CNS, the penetration of which are selectively limited by P-gp and Bcrp, respectively,7 were similar between WT and Mrp4-/- mice (Fig. 3), supporting the assertion that an Mrp4 defect does not affect P-gp and Bcrp activities at the BBB.
We then examined the impact of Mrp4 knockout on the concentrations in the brain after a 19 h infusion using an osmotic pump implanted under the skin in the backs of mice. In addition to the MRP4 substrates, the activities of which were confirmed using MRP4 membrane vesicles (Fig. 1 and Table 1), we added cyclophosphamide and ciprofloxacin as test substrates since MRP4-expressing HepG2 cells are resistant to cyclophosphamide,24 and mRNA of MRP4 and MRP2 were induced in the ciprofloxacin-resistant J774 macrophages.25 At the end of infusion, the plasma concentrations did not show a significant change between WT and Mrp4-/- mice. The Kp,brain values of most of the test drugs in WT mice were similar or somewhat greater than the vascular volume of WT mice (10 μL/g)26 in the brain, whereas ciprofloxacin, hydrochlorothiazide, and cyclophosphamide showed a relatively large distribution volume (Table 2). Among the test drugs, the Kp,brain values of ciprofloxacin, cyclophosphamide, methotrexate, and raltitrexed were higher in Mrp4-/- mice than in WT mice, although the difference for ciprofloxacin was not statistically significant (Table 2). For methotrexate and raltitrexed, we conducted CSF sampling at the end of experiment and determined that CSF concentrations of both drugs were also higher in the knockout mice (Table 2) because of higher Kp,brain accompanied with an increase in the flux from the CNS side to the CSF, and/or an increased permeability across the blood–CSF barrier.
In terms of substrate specificity, methotrexate is also the substrate of BCRP; however, there is little difference in the Kp,brain of methotrexate in Bcrp-/- mice.27 However, the urinary clearance of methotrexate in Bcrp-/- mice was reported to be decreased compared in WT mice.28 It was demonstrated that P-gp confers methotrexate resistance by carrier-mediated methotrexate uptake.29 We considered that inhibition of P-gp and Bcrp in Mrp4-/- mice may exhibit a marked increase in the brain concentration of methotrexate, greater than that available in the defect of Mrp4, as observed for the common substrate of P-gp and Bcrp in the P-gp/Bcrp double knockout mice.7 To inhibit P-gp and BCRP, GF120918, a dual inhibitor for P-gp and Bcrp,23 was administered. The Kp,kidney values of methotrexate and raltitrexed were higher in GF120918-treated mice, consistent with the important role of Bcrp in the kidney.28 Nevertheless, GF120918 was unexpectedly unable to elucidate the contribution of the efflux transporters at the BBB (Fig. 5). It has been demonstrated that Bcrp/Mrp4 double knockout mice had a significantly higher Kp,brain value than WT mice (2.1-fold).27 Future studies using triple knockout mice lacking P-gp, Bcrp, and Mrp4 should uncover the impact of P-gp and Bcrp on the brain concentrations of anionic drugs.
GF120918 decreased the tissue-to-plasma ratio of both methotrexate and raltitrexed, both in the cerebrum and cerebellum of Mrp4-/- mice, whereas it did not affect the corresponding value in the WT mice (Fig. 5). The effect of GF120918 can be interpreted as inhibition of the influx transporter at the BBB that can compete with the enhanced BBB permeability in Mrp4-/- mice. The transporter for the uptake of these folate analogs at the BBB remains unknown. One of the candidates is Oatp1a4, which is localized on the luminal membrane of the mouse (BBB).21 In fact, both methotrexate and raltitrexed were found to be Oatp1a4 substrates in complementary DNA (cDNA) transfected cells. Furthermore, the uptake was decreased along with the increase in GF120918 concentrations (Fig. 6). However, such an inhibitory effect was less for mOat3 than for mOatp1a4. The inhibitory effect of GF120918 is not common for multispecific organic anion transporters.
Fig. 6. Uptake into (A) mOatp1a4-expressing and mock cells of [3H]taurocholate (1 μM), [3H]methotrexate (50 nM), and raltitrexed (10 μM) and (B) mOat3-expressing and mock cells of [3H]estrone sulfate (0.1 μM), [3H]methotrexate (50 nM), and raltitrexed (10 μM) was determined after 2 min incubation in the presence or absence of probenecid or GF120918.
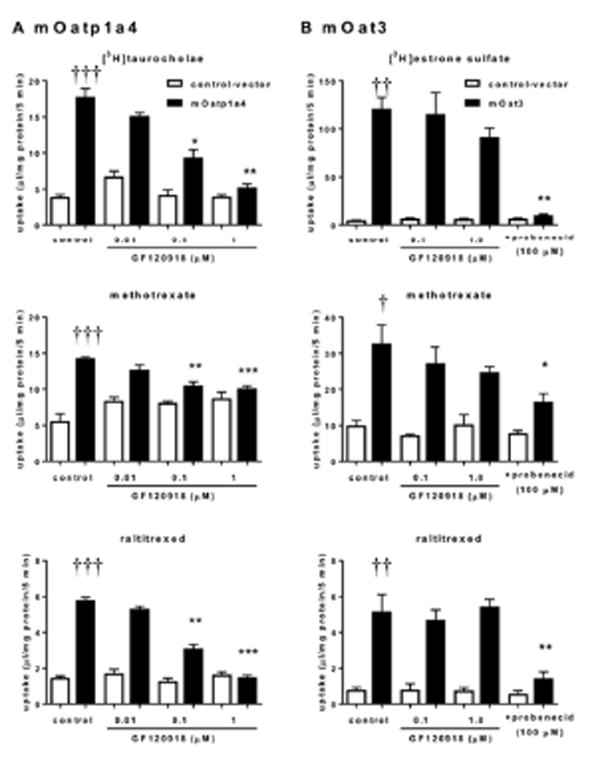
■, data for mOatp1a4 and mOat3-expressing cells; □, data for mock cells. Each bar with an error bar represents the mean value and S.E. (n = 3). Daggers represent statistically significant differences between mock and Oatp1a4 (or Oat3)-expressing cells in control conditions; †, P < 0.05; ††,P < 0.005; †††, P < 0.0005. Asterisks represent statistically significant differences between in the absence and presence of inhibitors in transporter expressing cells; *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
The present study reported that Mrp4 limits the penetration of methotrexate and raltitrexed into the cortex, cerebellum and CSF, and the penetration of cyclophosphamide into the brain in mice. Based on the in vivo data, we speculated that methotrexate and raltitrexed showed enhanced BBB permeability in Mrp4-/- mice because of the significant contribution of the uptake transporter that can be inhibited by GF120918 (Fig. 5). In fact, MRP4 inhibitors can target other ABC transporters.30 Moreover, vandetanib, a tyrosine kinase inhibitor, inhibited not only P-gp and BCRP but also OATP1B1 and OATP1B3 by everolims.31 Vandetanib also enhanced the plasma concentration of digoxin by the inhibition of P-gp and OATP1B3 transport.32 On the other hand, other MRP4 substrates, such as pitavastatin and pravastatin, did not show enhanced Kp,brain, despite their activities by MRP4 being similar or rather greater than methotrexate and raltitrexed. Furthermore, we reported that Oatp1a4 makes a significant contribution of the uptake by in situ brain perfusion.21 The reason for this discrepancy is unknown, although there are three possibilities. One is that the efflux transporters differentially contribute to the net efflux of organic anions across the luminal membrane. The second possibility is that the folate transport system facilitates the penetration of methotrexate and raltitrexed into the CNS. In fact, methotrexate is also recognized by folate transporters such as proton coupled folate transporter (PCFT/SLC46A1) and reduced folate carrier (RFC/SLC19A1), and raltitrexed inhibits these transporters, suggesting interaction between them.33 According to the database,34,35 the expression of PCFT in the mouse brain capillary endothelial cells is fairly low, whereas RFC is expressed to some degree. The third possibility is that the efflux across the abluminal membrane might be limited for other anionic drugs. Further studies are required to determinethe transport mechanisms of methotrexate and raltitrexed across the BBB.
The application of the present findings to humans remains debatable as the database search suggests the possibility of a species difference in the expression of MRP4 in the BBB between humans and mice; in the human brain, MRP4 is highly expressed in the microglia but to a low level in the endothelial cells. Aided by some uptake transporters, methotrexate and raltitrexed may penetrate and cross the BBB to reach primary tumors as well as metastases in the CNS for treatment in humans.
In conclusion, the penetration of methotrexate, raltitrexed, and cyclophosphamide mediated by some uptake transporters into the brain is limited by Mrp4 at the BBB in mice. However, not all MRP4 substrates showed enhanced penetration.
Acknowledgments
The authors would like to thank Daiichi Sankyo. Co., Ltd. for the gift of [3H]pravastatin and unlabeled pravastatin. We thank Hospira UK Limited and GlaxoSmithKline for the gift of raltitrexed, and GF120918 (Elacridar), respectively. We acknowledge supports (JDS) NIH grants R01CA194057,R01CA194206, P30 CA21745, CA21865, CA096832 and by ALSAC.
This study was supported by the Japan Society for the Promotion of Science; Grant-in-Aid for Scientific Research (B) [26293032].
Abbreviations
- ABC
ATP-binding cassette
- AMP
Adenosine monophosphate
- ATP
adenosine triphosphate
- BBB
blood–brain barrier
- BCRP
breast cancer resistance protein
- cDNA
complementary DNA
- cGMP
cyclic guanosine monophosphate
- CNS
central nervous system
- CSF
cerebrospinal fluid
- DHEAS
dehydroepiandrosterone sulfate
- Kp,tissue
tissue-to-plasma concentration ratio
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- MDR
multidrug resistance protein
- mRNA
messenger RNA
- MRP (Mrp in mice)
multidrug resistance-associated protein
- OAT (Oat in mice)
organic anion transporter
- OATP (Oatp in mice)
Organic anion transporting polypeptide
- PCFT
proton coupled folate transporter
- P-gp
P-glycoprotein
- RFC
reduced folate carrier
- S.E
standard error
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kusuhara H, Sugiyama Y. Active efflux across the blood-brain barrier: role of the solute carrier family. NeuroRx. 2005;2(1):73–85. doi: 10.1602/neurorx.2.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scherrmann JM. Expression and function of multidrug resistance transporters at the blood-brain barriers. Expert Opin Drug Metab Toxicol. 2005;1(2):233–246. doi: 10.1517/17425255.1.2.233. [DOI] [PubMed] [Google Scholar]
- 3.Schinkel AH. P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv Drug Deliv Rev. 1999;36(2–3):179–194. doi: 10.1016/s0169-409x(98)00085-4. [DOI] [PubMed] [Google Scholar]
- 4.Leggas M, Adachi M, Scheffer GL, Sun D, Wielinga P, Du G, Mercer KE, Zhuang Y, Panetta JC, Johnston B, Scheper RJ, Stewart CF, Schuetz JD. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol. 2004;24(17):7612–7621. doi: 10.1128/MCB.24.17.7612-7621.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enokizono J, Kusuhara H, Sugiyama Y. Effect of breast cancer resistance protein (Bcrp/Abcg2) on the disposition of phytoestrogens. Mol Pharmacol. 2007;72(4):967–975. doi: 10.1124/mol.107.034751. [DOI] [PubMed] [Google Scholar]
- 6.Enokizono J, Kusuhara H, Sugiyama Y. Quantitative investigation of the role of breast cancer resistance protein (Bcrp/Abcg2) in limiting brain and testis penetration of xenobiotic compounds. Drug Metab Dispos. 2008;36(6):995–1002. doi: 10.1124/dmd.107.019257. [DOI] [PubMed] [Google Scholar]
- 7.Kodaira H, Kusuhara H, Ushiki J, Fuse E, Sugiyama Y. Kinetic analysis of the cooperation of P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp/Abcg2) in limiting the brain and testis penetration of erlotinib, flavopiridol, and mitoxantrone. J Pharmacol Exp Ther. 2010;333(3):788–96. doi: 10.1124/jpet.109.162321. [DOI] [PubMed] [Google Scholar]
- 8.Borst P, de Wolf C, van de Wetering K. Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Arch. 2007;453(5):661–673. doi: 10.1007/s00424-006-0054-9. [DOI] [PubMed] [Google Scholar]
- 9.Belinsky MG, Guo P, Lee K, Zhou F, Kotova E, Grinberg A, Westphal H, Shchaveleva I, Klein-Szanto A, Gallo JM, Kruh GD. Multidrug resistance protein 4 protects bone marrow, thymus, spleen, and intestine from nucleotide analogue-induced damage. Cancer Res. 2007;67(1):262–268. doi: 10.1158/0008-5472.CAN-06-2680. [DOI] [PubMed] [Google Scholar]
- 10.Imaoka T, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the anti-viral drugs adefovir and tenofovir. Mol Pharmacol. 2007;71(2):619–627. doi: 10.1124/mol.106.028233. [DOI] [PubMed] [Google Scholar]
- 11.Ose A, Ito M, Kusuhara H, Yamatsugu K, Kanai M, Shibasaki M, Hosokawa M, Schuetz JD, Sugiyama Y. Limited brain distribution of [3R,4R,5S]-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate phosphate (Ro 64-0802), a pharmacologically active form of oseltamivir, by active efflux across the blood-brain barrier mediated by organic anion transporter 3 (Oat3/Slc22a8) and multidrug resistance-associated protein 4 (Mrp4/Abcc4) Drug Metab Dispos. 2009;37(2):315–321. doi: 10.1124/dmd.108.024018. [DOI] [PubMed] [Google Scholar]
- 12.Russel FG, Koenderink JB, Masereeuw R. Multidrug resistance protein 4 (MRP4/ABCC4): a versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol Sci. 2008;29(4):200–7. doi: 10.1016/j.tips.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Chen ZS, Lee K, Walther S, Raftogianis RB, Kuwano M, Zeng H, Kruh GD. Analysis of methotrexate and folate transport by multidrug resistance protein 4 (ABCC4): MRP4 is a component of the methotrexate efflux system. Cancer Res. 2002;62(11):3144–3150. [PubMed] [Google Scholar]
- 14.Nozaki Y, Kusuhara H, Kondo T, Iwaki M, Shiroyanagi Y, Nakayama H, Horita S, Nakazawa H, Okano T, Sugiyama Y. Species difference in the inhibitory effect of nonsteroidal anti-inflammatory drugs on the uptake of methotrexate by human kidney slices. J Pharmacol Exp Ther. 2007;322(3):1162–1170. doi: 10.1124/jpet.107.121491. [DOI] [PubMed] [Google Scholar]
- 15.Tian Q, Zhang J, Chan SY, Tan TM, Duan W, Huang M, Zhu YZ, Chan E, Yu Q, Nie YQ, Ho PC, Li Q, Ng KY, Yang HY, Wei H, Bian JS, Zhou SF. Topotecan is a substrate for multidrug resistance associated protein 4. Curr Drug Metab. 2006;7(1):105–118. doi: 10.2174/138920006774832550. [DOI] [PubMed] [Google Scholar]
- 16.Yamada A, Maeda K, Kamiyama E, Sugiyama D, Kondo T, Shiroyanagi Y, Nakazawa H, Okano T, Adachi M, Schuetz JD, Adachi Y, Hu Z, Kusuhara H, Sugiyama Y. Multiple human isoforms of drug transporters contribute to the hepatic and renal transport of olmesartan, a selective antagonist of the angiotensin II AT1-receptor. Drug Metab Dispos. 2007;35(12):2166–2176. doi: 10.1124/dmd.107.017459. [DOI] [PubMed] [Google Scholar]
- 17.Uchida Y, Kamiie J, Ohtsuki S, Terasaki T. Multichannel liquid chromatography-tandem mass spectrometry cocktail method for comprehensive substrate characterization of multidrug resistance-associated protein 4 transporter. Pharm Res. 2007;24(12):2281–2296. doi: 10.1007/s11095-007-9453-7. [DOI] [PubMed] [Google Scholar]
- 18.Ci L, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. Involvement of MRP4 (ABCC4) in the luminal efflux of ceftizoxime and cefazolin in the kidney. Mol Pharmacol. 2007;71(6):1591–1597. doi: 10.1124/mol.106.031823. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa M, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. Multidrug resistance–associated protein 4 is involved in the urinary excretion of hydrochlorothiazide and furosemide. J Am Soc Nephrol. 2007;18(1):37–45. doi: 10.1681/ASN.2005090966. [DOI] [PubMed] [Google Scholar]
- 20.Ose A, Kusuhara H, Endo C, Tohyama K, Miyajima M, Kitamura S, Sugiyama Y. Functional characterization of mouse organic anion transporting peptide 1a4 in the uptake and efflux of drugs across the blood-brain barrier. Drug Metab Dispos. 2010;38(1):168–176. doi: 10.1124/dmd.109.029454. [DOI] [PubMed] [Google Scholar]
- 21.Ose A, Kusuhara H, Yamatsugu K, Kanai M, Shibasaki M, Fujita T, Yamamoto A, Sugiyama Y. P-glycoprotein restricts the penetration of oseltamivir across the blood-brain barrier. Drug Metab Dispos. 2008;36(2):427–434. doi: 10.1124/dmd.107.018556. [DOI] [PubMed] [Google Scholar]
- 22.Deguchi T, Kusuhara H, Takadate A, Endou H, Otagiri M, Sugiyama Y. Characterization of uremic toxin transport by organic anion transporters in the kidney. Kidney Int. 2004;65(1):162–174. doi: 10.1111/j.1523-1755.2004.00354.x. [DOI] [PubMed] [Google Scholar]
- 23.Allen JD, Brinkhuis RF, Wijnholds J, Schinkel AH. The mouse Bcrp1/Mxr/Abcp gene: amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin. Cancer Res. 1999;59(17):4237–4241. [PubMed] [Google Scholar]
- 24.Tian Q, Zhang J, Tan TM Chan E, Duan W, Chan SY, Boelsterli UA, Ho PC, Yang H, Bian JS, Huang M, Zhu YZ, Xiong W, Li X, Zhou S. Human multidrug resistance associated protein 4 confers resistance to camptothecins. Pharm Res. 2005;22(11):1837–1853. doi: 10.1007/s11095-005-7595-z. [DOI] [PubMed] [Google Scholar]
- 25.Marquez B, Caceres NE, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. Identification of the efflux transporter of the fluoroquinolone antibiotic ciprofloxacin in murine macrophages: studies with ciprofloxacin-resistant cells. Antimicrob Agents Chemother. 2009;53(6):2410–2416. doi: 10.1128/AAC.01428-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dagenais C, Rousselle C, Pollack GM, Scherrmann JM. Development of an in situ mouse brain perfusion model and its application to mdr1a P-glycoprotein-deficient mice. J Cereb Blood Flow Metab. 2000;20(2):381–386. doi: 10.1097/00004647-200002000-00020. [DOI] [PubMed] [Google Scholar]
- 27.Sane R, Wu SP, Zhang R, Gallo JM. The effect of ABCG2 and ABCC4 on the pharmacokinetics of methotrexate in the brain. Drug Metab Dispos. 2014;42(4):537–40. doi: 10.1124/dmd.113.055228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vlaming ML, Pala Z, van Esch A, Wagenaar E, de Waart DR, van de Wetering K, van der Kruijssen CM, Oude Elferink RP, van Tellingen O, Schinkel AH. Functionally overlapping roles of Abcg2 (Bcrp1) and Abcc2 (Mrp2) in the elimination of methotrexate and its main toxic metabolite 7-hydroxymethotrexate in vivo. Clin Cancer Res. 2009;15(9):3084–3093. doi: 10.1158/1078-0432.CCR-08-2940. [DOI] [PubMed] [Google Scholar]
- 29.de Graaf D, Sharma RC, Mechetner EB, Schimke RT, Roninson IB. P-glycoprotein confers methotrexate resistance in 3T6 cells with deficient carrier-mediated methotrexate uptake. Proc Natl Acad Sci U S A. 1996;93(3):1238–42. doi: 10.1073/pnas.93.3.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huynh T, Norris MD, Haber M, Henderson MJ. ABCC4/MRP4: a MYCN-regulated transporter and potential therapeutic target in neuroblastoma. Front Oncol. 2012;2:178. doi: 10.3389/fonc.2012.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khurana V, Minocha M, Pal D, Mitra AK. Role of OATP-1B1 and/or OATP-1B3 in hepatic disposition of tyrosine kinase inhibitors. Drug Metabol Drug Interact. 2014;29(3):179–90. doi: 10.1515/dmdi-2013-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khurana V, Minocha M, Pal D, Mitra AK. Inhibition of OATP-1B1 and OATP-1B3 by tyrosine kinase inhibitors. Drug Metabol Drug Interact. 2014;29(4):249–59. doi: 10.1515/dmdi-2014-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao R, Qiu A, Tsai E, Jansen M, Akabas MH, Goldman ID. The proton-coupled folate transporter: impact on pemetrexed transport and on antifolates activities compared with the reduced folate carrier. Mol Pharmacol. 2008;74(3):854–862. doi: 10.1124/mol.108.045443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.http://web.stanford.edu/group/barres_lab/brainseqMariko/brainseq2.html
- 35.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34(36):11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


