Fig. 6. Stn1-Ten1 promotes DNA synthesis at telomeres to limit ssDNA accumulation.
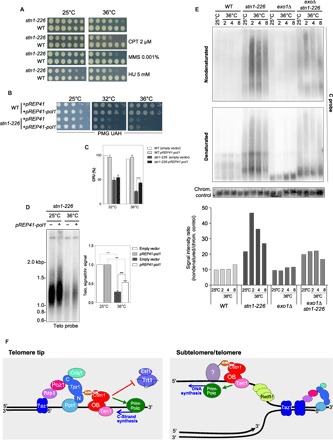
(A) WT and stn1-226 cells were grown at 25°C, then serially diluted (fivefold), and plated on YES-rich medium in the presence of the indicated drugs. (B) WT and stn1-226 cells containing pREP41 (empty vector) or pREP41-Pol1 plasmids were grown at 25°C in selective medium (PMG UAH), then serially diluted (fivefold), and plated. Plates were incubated at 25°, 32°, and 36°C. (C) Plating efficiencies were measured for WT and stn1-226 cells containing pREP41 (empty vector) or pREP41-Pol1 plasmids. Cells were grown at 25°C in selective medium and enumerated, and approximately a thousand cells were plated in triplicate. Plates were incubated at 25°, 32°, and 36°C. The plating efficiency was calculated by determining the ratio of CFU at 32° and 36°C above CFU at 25°C. Error bars indicate the SEM from multiple independent experiments (n = 4); P values are calculated from two-tailed t test (****P < 0.0001). (D) Overexpression of Pol1 partially complements stn1-226 defects. stn1-226 strains containing either pREP41 (empty vector) or pREP41-Pol1 were cultivated at 25°C in selective medium (PMG UAH) and then shifted at 36°C for 24 hours. Genomic DNA was prepared, digested with Eco RI restriction enzyme, and analyzed by Southern blotting with radiolabeled telomeric and chromosomic probes. Quantification of telomeric signal over chromosomic signal is presented. Error bars indicate the SEM from multiple independent experiments (n = 3); P values are calculated from two-tailed t test (***P < 0.005). (E) Analysis of Nsi I–digested genomic DNA was performed using in-gel nondenaturing hybridization, using a telomeric C probe. The same gel was denatured and rehybridized with the same probe and then hybridized again with a chromosomic probe (loading control). The G-strand signal intensities were quantified, and the ratio of intensities for nondenatured signal over denatured loading signal was calculated and plotted. (F) Dual action of Stn1-Ten1 complex at telomeres. Left: The Stn1-Ten1 complex acts as a modulator of telomerase action through the SIM domain of Stn1 and likely promotes the synthesis of the complementary strand by facilitating Polα action at telomere extremities. Right: At the subtelomeric and telomeric regions, the slowdown of the replication fork may accentuate the accumulation of nascent ssDNA. Thus, accessory factors are required to promote the replications of telomeres. The Stn1-Ten1 complex is likely required to either initiate or facilitate de novo DNA synthesis by the Prim-Polα complex. The interaction of Stn1 with a sumoylated partner through its SIM domain remains hypothetical. Stn1-Ten1 acts in a pathway that is independent of Taz1 and also distinct from Rad51, which might be required to protect nascent ssDNA at stalled fork at telomeres.
