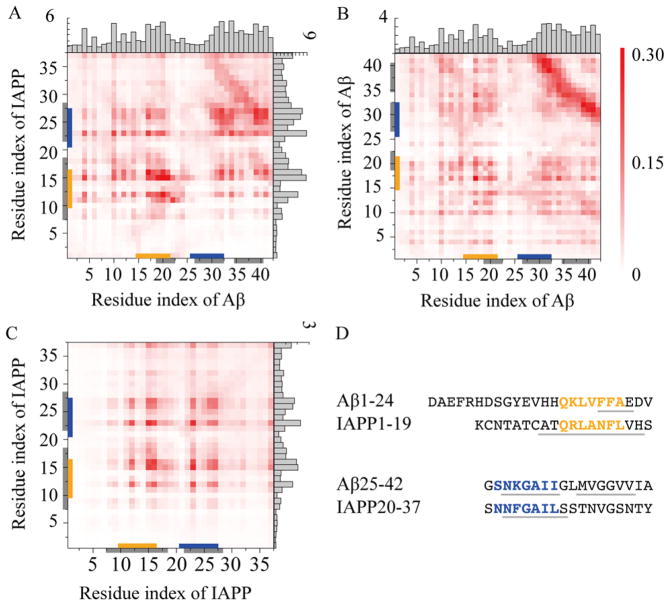
Figure 3.
Identification of hot regions for inter-peptide interactions in both cross- and self-associations of Aβ and IAPP. Residue-wise contact frequency maps were computed for (A) Aβ and IAPP binding in heterodimer simulations, and self-association of (B) Aβ and (C) IAPP in dimer simulations. Histograms were also obtained to show the total contact frequency of each residue. (D) Sequence fragments with the highest degree of similarity between Aβ and IAPP were highlighted in orange and blue, while the domains experimentally-identified to be important for both their cross- and self-associations were highlighted in gray.