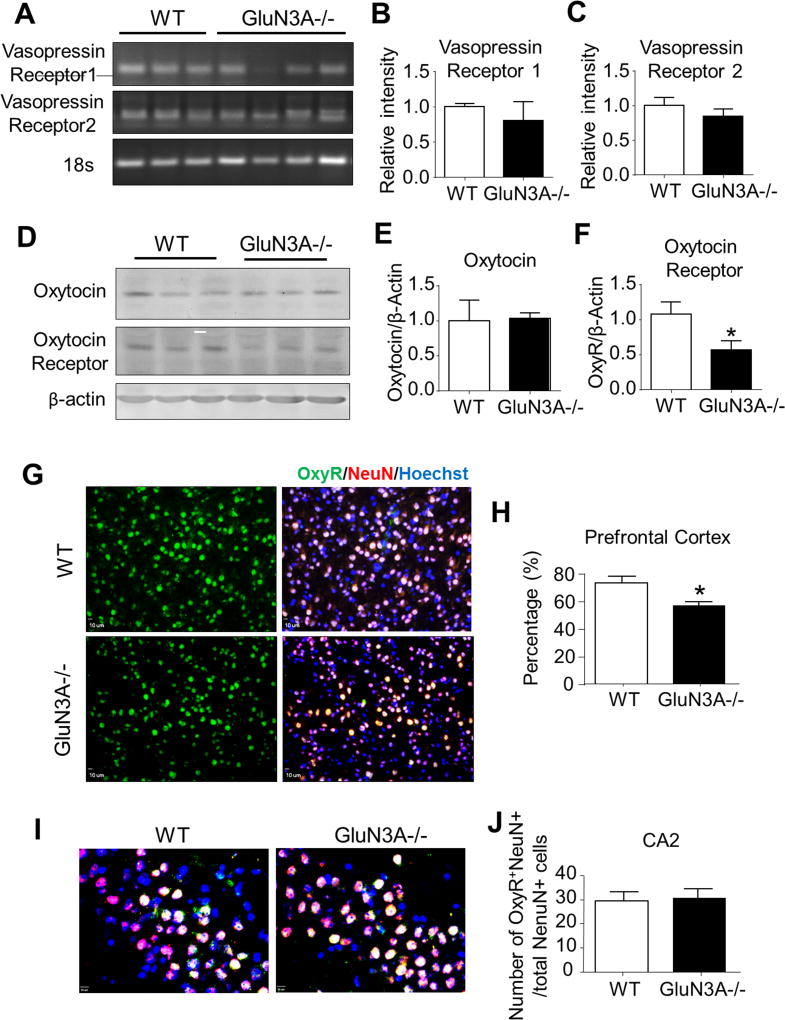
Figure 4. Deletion of GluN3A leads to abnormal development of oxytocin receptor.
We next performed RT-PCR analysis, Western blotting, and immunohistochemistry to investigate the possibility that the deletion of GluN3A could alter the development of the oxytocin system and vasopressin system. A. RT-PCR analysis of vasopressin (AVP) receptors 1 and 2 in the prefrontal cortex of WT and GluN3A KO mice. B and C. Quantification of relative intensity (normalized to 18S ribosomal RNA control) of AVP receptors 1 and 2 in the prefrontal cortex. Student t test; n=6 per group. D. Western blotting of oxytocin and oxytocin receptor in the prefrontal cortex from WT and GluN3A KO mice. E and F. Quantification of the optic density (normalized to β-actin control) of oxytocin and oxytocin receptor in the prefrontal cortex. Student t test; *P<0.05 vs. WT; n=6 per group. G. Immunohistochemistry analysis revealed that oxytocin receptor-positive cells were completely co-labeled with NeuN positive cells in the prefrontal cortex. H. In the prefrontal cortex, the ratio of oxytocin receptor (green)-NeuN (red) double-positive cells to total NeuN-positive cells was lower in GluN3A KO mice as compared to WT mice. Student t test; *P<0.05 vs. WT; n=4–5 per group. I. Immunohistochemistry analysis revealed oxytocin receptor-positive cells in CA2 area of hippocampus. Scale bars = 50 µM. J. The number of oxytocin receptor-NeuN double-positive cells was similar in GluN3A KO mice as compared to WT mice. Student t test; *P<0.05 vs. WT; n=4–5 per group.