Figure 1.
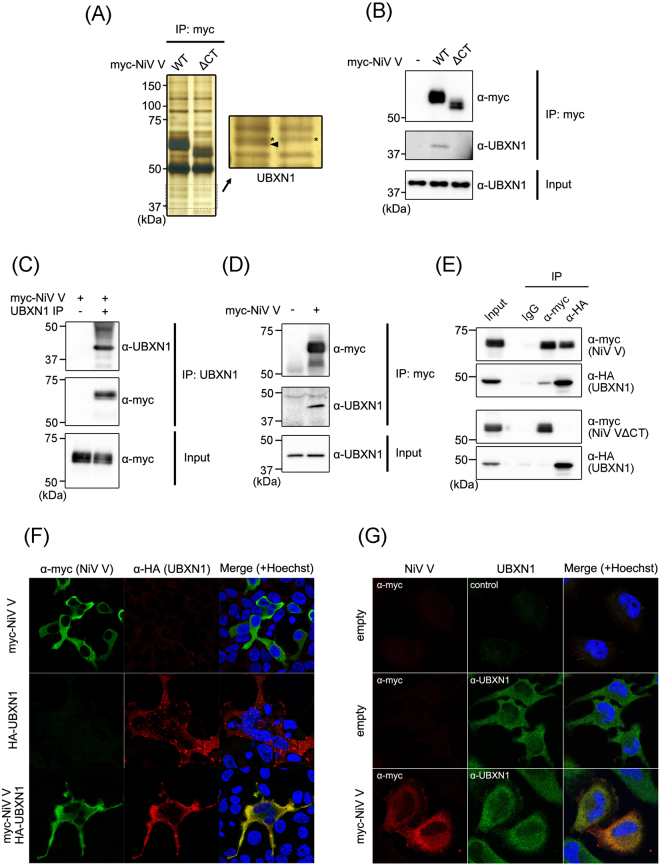
Identification of a protein that interacts with NiV V. (A) Myc-tagged NiV V and a mutant lacking the C-terminal domain (ΔCT) were expressed in HEK293T cells. At 48 h posttransfection, an immunoprecipitation assay was performed, and the precipitated proteins were detected with silver staining. The band indicated by the arrowhead was analyzed with mass spectrometry. *Nontargeted bands. (B) The immunoprecipitation assay was performed as described in (A), and the precipitated proteins were detected with western blotting. (C) Myc-tagged NiV V was expressed in HEK293T cells, and at 48 h posttransfection, an immunoprecipitation assay was performed using UBXN1-specific antibody. The precipitated proteins were detected with western blotting. (D) Myc-tagged NiV V expressed in HeLa cells was immunoprecipitated, and the precipitated proteins were detected with western blotting. (E) Myc-tagged NiV V or ΔCT were expressed together with HA-tagged UBXN1 in HEK293T cells, and after 48 h, an immunoprecipitation assay was performed with anti-myc or anti-HA antibody. The precipitated proteins were detected with western blotting. (F) NiV V and HA-tagged UBXN1 were expressed in HEK293T cells, and after 24 h, an indirect immunofluorescence assay was performed. The subcellular localization of NiV V and UBXN1 was observed with confocal microscopy. (G) NiV V was expressed in HeLa cells, and the subcellular localization of NiV V and endogenous UBXN1 was examined by an indirect immunofluorescence assay. The gel and blots presented in (A–E) were cropped from different images to improve clarity. Full-length gel and blots are presented in Supplementary Figure S1.