Abstract
Cisplatin, a widely used cancer therapy drug, induces nephrotoxicity or acute kidney injury (AKI), but the underlying mechanism remains unclear, and renal protective approaches are not available. Fibroblast growth factor (FGF)21 is an endocrine factor that regulates glucose uptake, metabolism, and energy expenditure. However, recent work has also implicated FGF21 in cellular stress response under pathogenic conditions. The role and regulation of FGF21 in AKI are unclear. Here, we show that FGF21 was dramatically induced during cisplatin treatment of renal tubular cells in vitro and mouse kidneys in vivo. The inductive response was suppressed by pifithrin (a pharmacological inhibitor of P53), suggesting a role of P53 in FGF21 induction. In cultured renal tubular cells, knockdown of FGF21 aggravated cisplatin-induced apoptosis, whereas supplementation of recombinant FGF21 was protective. Consistently, recombinant FGF21 alleviated cisplatin-induced kidney dysfunction, tissue damage, and tubular apoptosis in mice. Mechanistically, FGF21 suppressed P53 induction and activation during cisplatin treatment. Together, these results indicate that FGF21 is induced during cisplatin nephrotoxicity to protect renal tubules, and recombinant FGF21 may have therapeutic potential.—Li, F., Liu, Z., Tang, C., Cai, J., Dong, Z. FGF21 is induced in cisplatin nephrotoxicity to protect against kidney tubular cell injury.
Keywords: acute kidney injury, apoptosis, P53
Acute kidney injury (AKI) is a kidney disease characterized by the rapid decline of renal function, which is often caused by renal ischemia-reperfusion, nephrotoxins, and sepsis. The pathogenesis of AKI is multifactorial, involving multiple cell types, cellular processes, and molecular mediators and regulators (1). A critical pathologic feature of AKI is sublethal and lethal injury of the epithelial cells in renal tubules (2). In addition to its association with acute morbidity and mortality, AKI is an important risk factor for the development of chronic kidney diseases (CKDs) (3).
Cisplatin is one of the most widely used chemotherapeutic drugs, but it is also notorious for its side effects in normal tissues, especially nephrotoxicity in kidneys (4–6). The research in the past decade has demonstrated that cisplatin exposure results in DNA damage response, mitochondrial damage, and various stress responses in renal tubular cells, leading to tubular damages and renal dysfunction. P53, a well-known tumor suppression protein, has been demonstrated to play a key role in the pathogenesis of cisplatin-induced nephrotoxicity or AKI (7, 8). Under this condition, P53 is robustly activated in renal proximal tubular cells, and its activation may result in tubular cell death through transactivation of proapoptotic gene, such as p53 up-regulated modulator of apoptosis (PUMA-α) and p53-induced protein with a death domain (PIDD) (5, 9, 10). As a result, pharmacological and genetic inhibition of P53 is beneficial to cisplatin nephrotoxicity (11, 12).
Fibroblast growth factor (FGF)21 is an atypical member of the FGF family that lacks the heparin binding domain of typical FGF members and thus can be released into the circulation (13). FGF21 functions through binding to 1 of the FGF21 receptors via its N terminus and β-klotho via its C terminus. A main function of FGF21 is to regulate glucose and lipid metabolism to maintain the body’s energy metabolism balance (14, 15). Recent studies have revealed that FGF21 may also be a cell stress response factor. For instance, in sepsis, FGF21 acts as an acute phase response protein to protect against inflammation and oxidative stress (16, 17). FGF21 has also been implicated in the protection of cardiomyocytes, pancreatic islet cells, and vascular endothelial cells under conditions such as diabetic lipotoxicity (16–19).
In kidneys, FGF21 was shown to be protective in murine models of diabetic nephropathy and dyslipidemic nephropathy by reducing proteinuria excretion, suppressing mesangial expansion, and inhibiting fibrosis factors (20, 21). In humans, high FGF21 accompanied by endothelial dysfunction was detected in patients on long-term hemodialysis (22). Despite these observations, the function and regulation of FGF21 in the pathogenesis of AKI remains to be elucidated. In the present study, we have demonstrated FGF21 induction in proximal tubular cells during cisplatin-induced AKI. Silencing of FGF21 aggravated cisplatin-induced tubular cell injury, whereas supplementation of recombinant FGF21 protected against cisplatin nephrotoxicity. We also provide evidence that FGF21 induction in cisplatin-AKI may depend on P53 and that FGF21 can negatively regulate P53 activation. Together, these results support an important role for FGF21 in preserving tubular cell viability and function in cisplatin-AKI, suggesting the therapeutic potential of FGF2.
MATERIALS AND METHODS
Special reagents
The primary antibodies used were from the following sources: anti–phospho-(serine-15)-P53, anti-P53, and anti–cleaved caspase-3 (C-caspase 3) antibodies were from Cell Signaling Technology (Danvers, MA, USA); anti-FGF21 antibody was from Abcam (Cambridge, United Kingdom); anti–KIM-1 antibody was from Abcam; anti–β-actin was from Sigma-Aldrich (St. Louis, MO, USA); all secondary antibodies for immunoblot analysis were from Thermo Fisher Scientific (Waltham, MA, USA). Cisplatin and pifithrin-α were purchased from Sigma-Aldrich. Recombinant mouse FGF21 protein was from Genscript Biotech (Piscataway, NJ, USA).
Mouse model of cisplatin nephrotoxicity
Male C57BL/6 mice (8 wk of age) purchased from Hunan Slack King Experimental Animal company (Changsha, China) were injected intraperitoneally with a single dose of 30 mg/kg body weight cisplatin. Control mice were injected with a comparable volume of saline. To study the effects of FGF21, recombinant FGF21 was dissolved in PBS and administered intraperitoneally at 0.1 μg/g body weight 1 h before cisplatin injection followed by injection every 8 h. A comparable volume of PBS was given to the no-FGF21 animals. Mice were euthanized at d 3. All animal experiments were performed according to a protocol approved by the Institutional Committee for the Care and Use of Laboratory Animals of Second Xiangya Hospital at Central South University.
Cells and cisplatin treatment
The Boston University mouse proximal tubular (BUMPT) cell line (BUMPT-306) was originally obtained from Drs. William Lieberthal and John Shwartz at Boston University (23). The cells were cultured in DMEM with 10% fetal bovine serum and 10% streptomycin. FGF21 short hairpin RNA (shRNA) plasmids pLKO.1-FGF21 and control vector pLKO.1-puro plasmid were generous gifts from Dr. Jinke Cheng (Shanghai Jiao Tong University School of Medicine, Shanghai, China). The shRNA sequences are KD1 (forward: 5′-CCGGGAT GACGACCAAGACACTGAACTCGAGTTCAGTGTCTTGGTCGTCATCTTTTTC-3′, reverse: 5′-AATTGAAAAAGATGACGACCAAGACACTGAACTCGAGTTCAGTGTCTTGGTCGTCATC-3′) and KD2 (forward: 5′-CCGGTCTCTATGGATCGCCTCACTTCTCGAGAAGTGAGGCGATCCATAGAGATTTTTC-3′, reverse: 5′-AATTGAAAAATCTCTATGGATCGCCTCACTTCTCGAGAAGTGAGGCGATCCATAGAGA-3′). The plasmids were packaged in 293T cells together with psPAX2 and pMD2.G (Addgene, Cambridge, MA, USA). Medium was changed 6 h later, and lentiviral particles were collected at 48 h after Lipo2000 (Invitrogen, Carlsbad, CA, USA) transfection. The medium was filtered through a 0.45-μm filter and used to infect BUMPT cells with 8 μg/ml polybrene for 48 h. Infected cells were selected with 2.5 μg/ml puromycin (Sigma-Aldrich) for 7–10 d. The cells were collected, and the efficiency of knockdown was determined by immunoblotting. The stably transfected cells were grown in DMEM supplemented with 10% fetal bovine serum and 10% streptomycin. For cisplatin treatment, BUMPT cells were incubated with 20 μM cisplatin, which induced significant apoptosis as previously indicated (23).
Examination of apoptosis in cell cultures and kidneys
Apoptosis in cell cultures was analyzed by cell morphology. For morphologic analysis, cells were stained with Hoechst33342. The cells showing typical morphologic features, including cellular and nuclear condensation and fragmentation, were counted to determine the percentage of apoptosis. Apoptosis in kidney tissues was analyzed by TUNEL assay using the in situ Cell Death Detection Kit from Roche Diagnostics (Indianapolis, IN, USA), as described in our previous work (24, 25).
Analysis of renal function and histology
Renal function was measured by serum creatinine and BUN using commercial kits (Bioassay System, Hayward, CA, USA) according to the manufacturer’s instructions. For histology, kidney tissues were fixed with 4% paraformaldehyde for paraffin embedding, hematoxylin and eosin staining, TUNEL assay, and immunohistochemistry. Tissue damage was scored by the percentage of renal tubules with cell lysis, loss of brush border, and cast formation (0, no damage; 1, <25%; 2, 25–50%; 3, 50–75%; 4, >75%).
Immunoblot analysis
Cells and kidney tissue were lysed in a buffer containing 2% SDS and protease inhibitor cocktail. Reducing gel electrophoresis and protein transferring/blotting were conducted by standard procedures. The blots were incubated with 5% bovine serum albumin for blocking and then exposed to specific primary antibodies overnight at 4°C. Finally, the blots were exposed to the horseradish peroxidase–conjugated secondary antibody, and antigens on the blots were revealed using an ECL Kit (EMD Millipore, Billerica, MA, USA).
Immunohistochemistry
Immunohistochemical staining for FGF21 was performed using a protocol described previously (25). Briefly, deparaffinized sections were placed in 0.1 M sodium citrate (pH 6.0) at 95–100°C for 1 h for antigen retrieval and sequentially incubated with 3% H2O2, blocking buffer (2% normal goat serum). The slides were then exposed to 1:200 anti-FGF21 (ab-171941) at 4°C overnight and biotinylated goat anti-mouse secondary antibody (VP-9000; Beijing Zhongshan Jinqiao Biotechnology, Beijing, China) for 20 min at room temperature. Negative controls were done by replacing the primary antibody with antibody diluent. The color was developed with a DAB kit (Vector Laboratories, Burlingame, CA, USA). From each section, 10–20 fields (original magnification, ×200) were randomly selected. The percentage of FGF21-positive stained area was quantified by ImageJ.
Statistical analyses
Qualitative data shown in this study, including immunoblots and cell and tissue images, are representative of at least 3 separate experiments. Quantitative data are expressed as means ± sd. A Student’s t test was used to determine the statistical significance in the differences between 2 groups, and 1-way ANOVA followed by Tukey’s post hoc test was used to compare multiple groups. Statistical analysis was performed using SPSS 17 software (IBM SPSS, Chicago, IL, USA). A value of P <0.05 was considered to reflect significant differences.
RESULTS
FGF21 is induced during cisplatin treatment in BUMPT cells
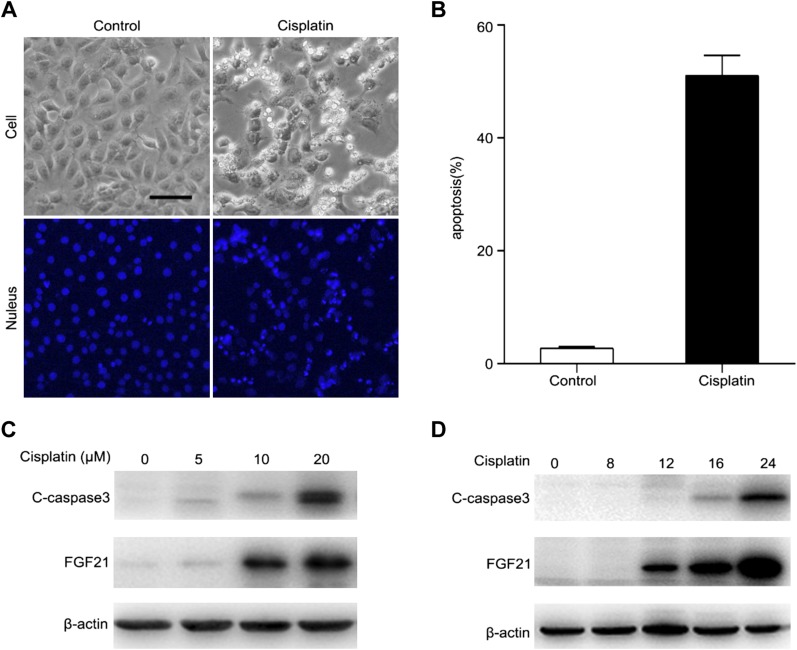
We initially examined the expression of FGF21 in the BUMPT cell line (23). after 20 μM cisplatin treatment for 24 h, some BUMPT cells showed the typical cellular and nuclear morphologies of apoptosis, including cellular shrinkage and blebbing, and nuclear condensation and fragmentation as visualized by Hoechst 33342 staining (Fig. 1A). Quantification by counting revealed that cisplatin induced ∼50% apoptosis in BUMPT cells (Fig. 1B). The morphologic assay results were confirmed by immunoblot analysis of C-caspase 3. Cisplatin induced the active form of C-caspase 3 in BUMPT cells in a concentration and treatment time–dependent manner (Fig. 1C, D). Treatment with 20 μM cisplatin for 24 h induced robust caspase-3 activation. FGF21 was up-regulated upon cisplatin treatment (Fig. 1C, D). The increase of FGF21 appeared to occur before the activation of caspase 3 (Fig. 1C, D). Collectively, these findings suggest that FGF21 is induced during cisplatin treatment of renal tubular cells.
Figure 1.
FGF21 is induced during cisplatin treatment in BUMPT cells. A) Representative cellular nuclear morphology. BUMPT cells were treated with cisplatin (20 μM) for 24 h and then stained with Hoechst 33342. Cellular and nuclear morphology were recorded by microscopy (original magnification, ×200). Scale bar, 50 μm. B) Apoptosis percentage. Fewer than 200 cells in each group were evaluated to determine the percentage of cells with typical apoptotic morphology. Means ± sd (n = 3). *P < 0.0001 vs. untreated control cells. C, D) BUMPT cells were treated with different concentration of cisplatin (0, 5, 10, and 20 μM) for 24 h (C) or with 20 μM cisplatin for 0, 8, 12, 16, or 24 h (D). Whole cell lysates were collected for immunoblot analysis of C-caspase 3, FGF21, and β-actin as loading control.
FGF21 is induced in kidney tissues after cisplatin-induced AKI
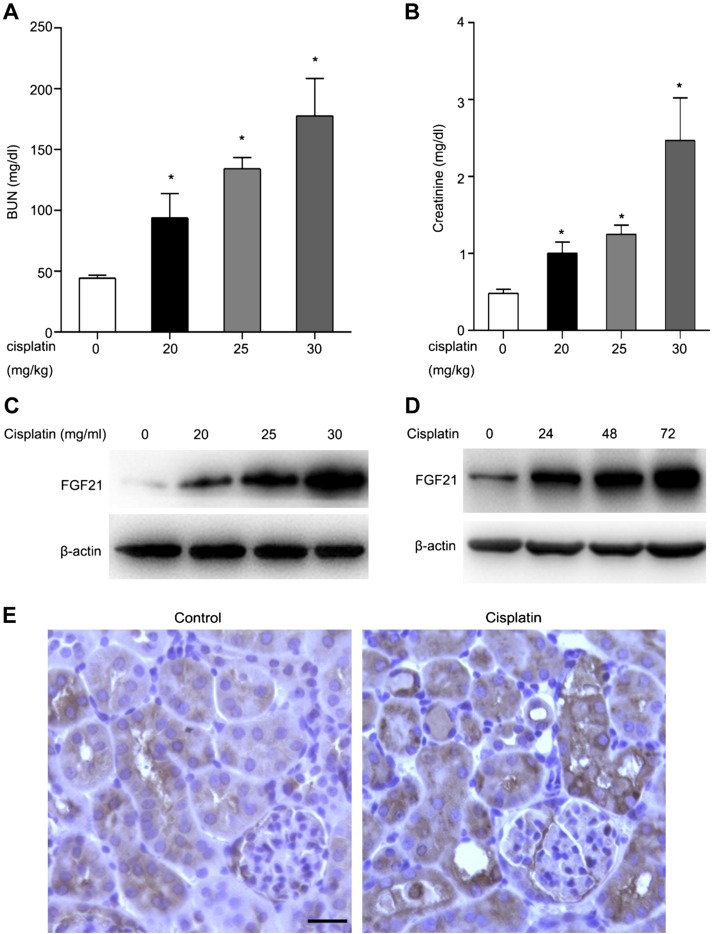
In vivo, we determined the expression of FGF21 in the kidney tissues of mouse model of cisplatin-induced AKI. To this end, C57Bl/6 mice (male, 8 to ∼10 wk old) were administered different dosages of cisplatin by intraperitoneal injection. Mice were euthanized at the indicated time points. Blood was collected for the measurement of serum creatinine and BUN. Cisplatin treatment for 24 h increased serum creatinine and BUN in a dosage-dependent manner (Fig. 2A, B). Immunoblot analysis revealed a dramatic induction of FGF21 expression in kidney tissues after cisplatin treatment, and the induction was cisplatin treatment dose- and time dependent (Fig. 2C, D). Further immunohistochemical analysis detected weak FGF21 signals in renal tubules, which was increased after cisplatin treatment, especially in the tubules with apparent injury (Fig. 2C). Collectively, these results demonstrate that FGF21 is induced in kidney tubular cells in cisplatin-induced AKI.
Figure 2.
FGF21 is induced in kidney tissues after cisplatin-induced AKI. C57Bl/6 mice (male, 8–10 wk old) were treated with cisplatin (20, 25, or 30 mg/kg body weight) and euthanized at 24, 48, or 72 h. A, B) Blood samples were collected for measurements of BUN (A) and serum creatinine (B). Means ± sd (n = 5). *P < 0.05 vs. untreated control group. C, D, E) Renal tissues were collected for histologic analysis and for immunoblot as well as immunohistochemical analysis of FGF21 expression. Scale bar, 50 μm. C, D) Representative immunoblotting of FGF21 and β-actin (loading control). E) Representative immunohistochemical staining of FGF21 within kidney tissues.
Suppression of cisplatin-induced FGF21 expression by pifithrin-α
P53 contributes critically to tubular cell injury and death in cisplatin-AKI, and the underlying mechanism involves the regulation of gene expression (11, 12). We hypothesized that P53 might regulate FGF21 expression in renal tubular cells during cisplatin nephrotoxicity. To test this hypothesis, we evaluated the effects of P53 inhibitor pifithrin-α on FGF21 expression during cisplatin treatment of BUMPT cells. BUMPT cells were treated with 20 μΜ cisplatin in the presence or absence of 20 μM pifithrin-α for 24 h. As expected from our previous work (9, 26), pifithrin-α reduced cisplatin-induced apoptosis in BUMPT cells (Fig. 3A, B). Immunoblot analysis of C-caspase 3 further confirmed the protective effect of pifithrin-α. Pifithrin-α dramatically reduced the levels of C-caspase 3 after cisplatin treatment (Fig. 3C). FGF21 induction by cisplatin was markedly abrogated by pifithrin-α treatment (Fig. 3C). These results suggest that FGF21 induction during cisplatin nephrotoxicity is largely P53 dependent.
Figure 3.
Suppression of cisplatin-induced FGF21 expression by pifithrin-α. BUMPT cells were treated with cisplatin (20 μM) in the presence or absence of pifithrin-α (20 μM). A) Representative images of cellular and nuclear morphology. Scale bar, 50 μm. B) Percentage of apoptosis. Means ± sd (n = 3). *P < 0.0005 vs. untreated control cells, #P < 0.005 vs. cisplatin-only treated KD group cells. C) Representative immunoblots of FGF21, p-P53, P53, C-caspase 3, and β-actin as protein loading control.
Silencing of FGF21 sensitizes BUMPT cells to cisplatin-induced apoptosis
To determine the function of FGF21 in renal tubular cells, we first assessed the effect of FGF21 silencing on cisplatin-induced apoptosis in BUMPT cells. To this end, we generated stable FGF21 knockdown (KD) cell lines by transfecting the pLKO.1-FGF21 shRNA plasmids KD1 and KD2 into BUMPT cells. The control group was transfected with the pLKO.1-puro control shRNA plasmid [negative control (NC)]. Both cell lines showed obvious KD effect (Fig. 4A), with more effect in KD2 cells. KD2 cells were used for further study due to their high knockdown efficacy. Apoptosis was minimal under control conditions regardless of the status of FGF21 (Fig. 4B). Upon cisplatin treatment, some cells showed the typical morphology of apoptosis in both NC and FGF21-KD groups (Fig. 4B). Quantification by counting revealed that cisplatin induced ∼50% apoptosis in NC cells but induced 70% apoptosis in FGF21-KD cells (Fig. 4C). The morphologic assay results were confirmed by immunoblot analysis of cleaved/active caspase-3. In response to cisplatin, FGF21KD cells showed significantly higher levels of C-caspase 3 that NC cells. Meanwhile, recombinant FGF21 reduced cisplatin-induced C-caspase 3 in NC and FGF21-KD2 cells (Fig. 4D). Collectively, these findings suggest that FGF21 plays a cytoprotective or prosurvival role during cisplatin treatment in renal tubular cells.
Figure 4.
Silencing of FGF21 sensitizes BUMPT cells to cisplatin-induced apoptosis. A) BUMPT stable cell lines were generated by transfecting shRNA pLKO.1-FGF21 plasmids (KD1, KD2) to silence endogenous FGF21 expression or by transfecting NC shRNA vector as control. KD efficiency was determined by immunoblot analysis of FGF21 and β-actin (loading control). B) BUMPT stable cell lines were treated with cisplatin (20 μM) for 24 h to record cell morphology. Scale bar, 100 μm. C) BUMPT stable cell lines were pretreated with or without recombinant FGF21 (50 ng/ml) for 1 h and cultured in the presence or absence of cisplatin (20 μM) for 24 h. Representative immunoblotting of C-caspase 3 and β-actin as protein loading control. D) Percentage of apoptosis. BUMPT stable cell lines were treated with cisplatin (20 μM) for 24 h to assess apoptosis by cell morphology. Means ± sd (n = 3). *P < 0.01 vs. cisplatin treated NC group cells.
Recombinant FGF21 decreases cisplatin-induced BUMPT cell apoptosis
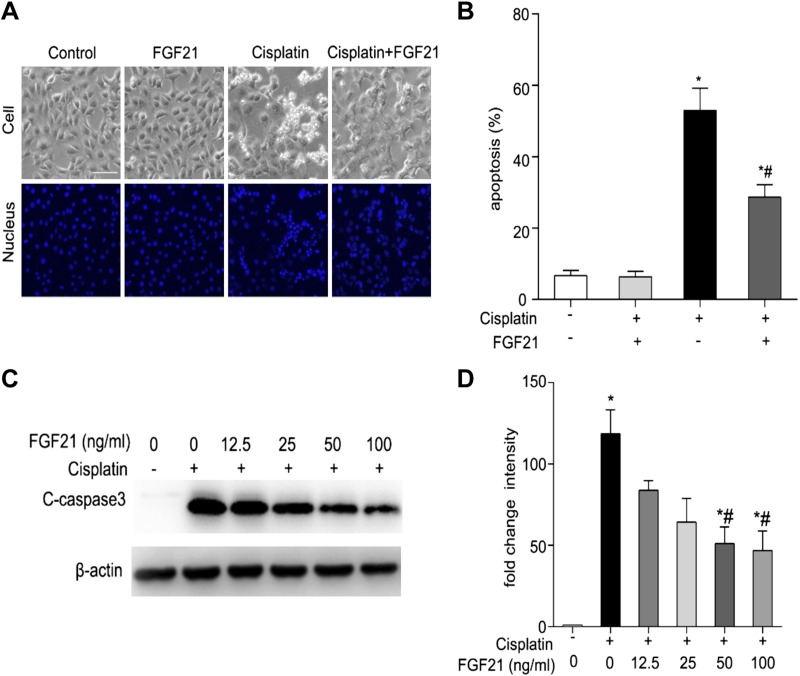
We determined whether the addition of exogenous FGF21 was beneficial to cisplatin nephrotoxicity. BUMPT cells were incubated with 20 μM of cisplatin in the presence or absence of 50 ng/ml of FGF21 for 24 h. Morphologic assays and immunoblot analysis of C-caspase 3 were performed to evaluate the effect of recombinant FGF21. Compared with the cells treated with cisplatin only, there were many fewer apoptotic cells in the group pretreated with recombinant FGF21 (Fig. 5A). Quantification by counting revealed that cisplatin induced ∼50% apoptosis, which was reduced to 30% by FGF21 (Fig. 5B). In line with the morphologic observation, immunoblot analysis showed that supplementation of FGF21 reduced the levels of C-caspase 3 in the BUMPT cells during cisplatin incubation, and the effect of FGF21 was dosage dependent (Fig. 5C, D). Collectively, these results provide further evidence that FGF21 has protective effects during cisplatin treatment of renal tubular cells.
Figure 5.
Recombinant FGF21 decreases cisplatin-induced BUMPT cell apoptosis. A, B) BUMPT cells were pretreated with or without recombinant FGF21 (50 ng/ml) for 1 h and cultured in the presence or absence of cisplatin (20 μM) for 24 h. Cells were stained with Hoechst 33342 to record cellular and nuclear morphology. A) Representative images of cellular and nuclear morphology. Scale bar, 100 μm. B) Percentage of apoptosis. Means ± sd (n = 3). *P < 0.0005 vs. untreated control cells; #P < 0.005 vs. cisplatin-only treated cells. C, D) BUMPT cells were pretreated with or without recombinant FGF21 (0, 12.5, 25, 50, and 100 ng/ml) for 1 h and incubated with cisplatin (20 μM) for 24 h. Whole cell lysates were collected for immunoblot analysis of C-caspase 3 and β-actin as protein loading control. C) Representative immunoblots. D) Densitometry of C-caspase 3 signals. The cleaved-caspase 3 signals were normalized to the β-actin signal of the same samples to determine the ratios. The ratios of control group (in the absence of cisplatin and FGF21) were arbitrarily set as 1. Means ± sd (n = 3). *P < 0.0001 vs. untreated control cells, #P < 0.001 vs. cisplatin-only–treated cells.
Recombinant FGF21 attenuates cisplatin-induced AKI in mice
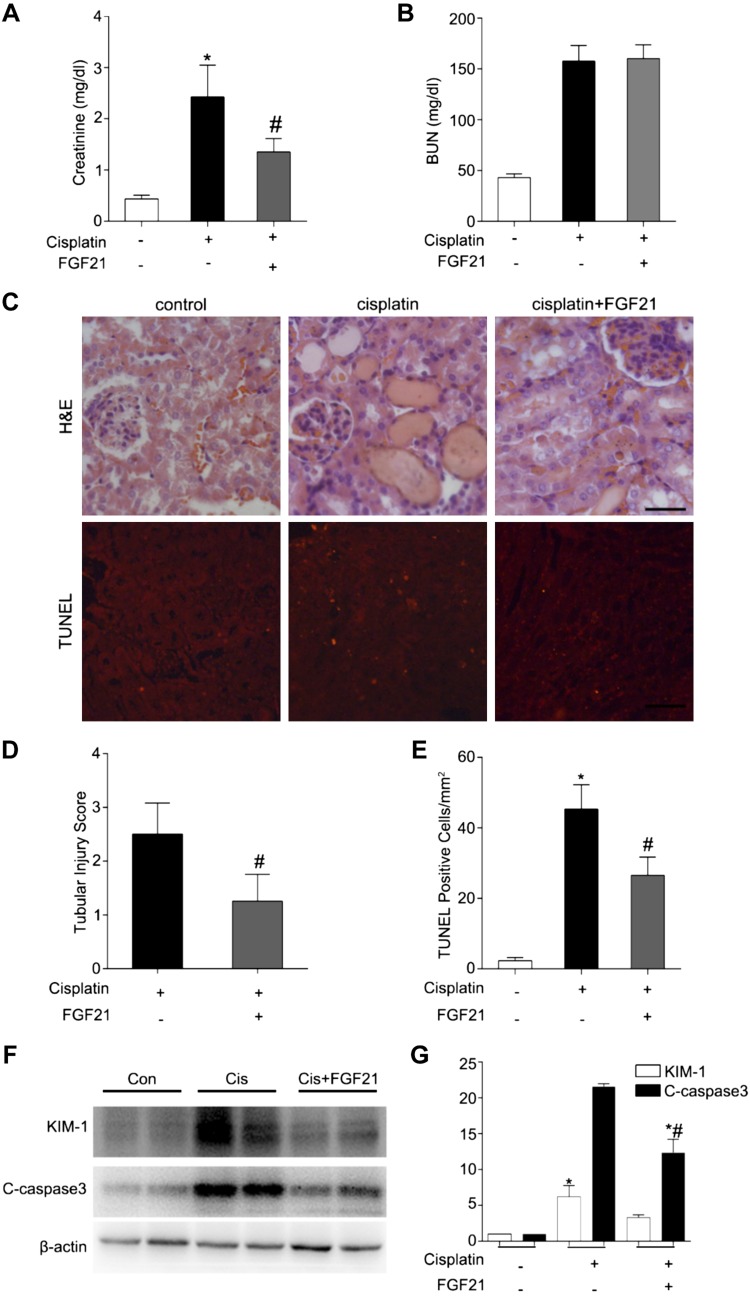
We determined whether supplementation of FGF21 is renoprotective during cisplatin nephrotoxicity in vivo. In this experiment, C57Bl/6 mice were intraperitoneally injected with FGF21 (0.1 μg/g) or saline 1 h before cisplatin (30 mg/kg) administration. After that, mice were administered FGF21 or saline every 8 h and were euthanized at d 3. Mice treated with both cisplatin and FGF21 had a significantly lower level of serum creatinine than those treated with cisplatin alone (Fig. 6A). However, these 2 groups of mice showed similar levels of BUN (Fig. 6B). We examined renal histology to verify the effect of FGF21. Mice treated with cisplatin plus FGF21 showed less severe tubular damage in comparison to the mice treated with cisplatin alone (Fig. 6C). The tubular injury score for cisplatin-only mice was ∼2.5, which was reduced to ∼1.2 in mice treated with FGF21 and cisplatin (Fig. 6D). In addition, TUNEL assay revealed that there was significantly less tubular cell apoptosis in mice treated with cisplatin and FGF21 compared with those treated with cisplatin only (Fig. 6C, E). We also detected the KIM-1 and C-caspase 3 by immunoblot. Furthermore, recombinant FGF21 alleviated KIM-1 and C-caspase 3 induced by cisplatin (Fig. 6F, G). Collectively, these in vivo findings provide compelling evidence for a protective role of FGF21 in cisplatin nephrotoxicity.
Figure 6.
Recombinant FGF21 attenuates cisplatin-induced AKI in mice. C57Bl/6 mice (male, 8–10 wk) were administered cisplatin (30 mg/kg) with or without pretreatment of FGF21 (0.1 μg/g) or with saline as control. Mice were euthanized at 24, 48, or 72 h. A, B) Blood samples were collected for measurements of serum creatinine (A) and BUN (B). C–E) Renal tissues were collected for histologic analysis and tubular cell death analysis. C) Upper panel: Representative histology of kidney cortex by hematoxylin and eosin (H&E) staining. Scale bar, 50 μM. Lower panel: Representative images of TUNEL staining of kidney tissues. Scale bar, 50 μM. D) Quantification of TUNEL-positive cells in kidney tissues. E) Histopathological score of tubular damage. F, G) Renal tissues were collected for immunoblot analysis of KIM-1, C-caspase 3, and β-actin as protein loading control. F) Representative immunoblotting. G) Densitometry of KIM-1 and C-caspase 3 signals. The KIM-1 and C-caspase 3 signals were normalized to the β-actin signal of the same samples to determine the ratios. Means ± sd (n = 4). *P < 0.01 vs. the control group (A, B, D, E), #P < 0.05 vs. cisplatin-only group; *P < 0.05 vs. the control group; #P < 0.01 vs. cisplatin-only group (G).
FGF21 suppresses P53 during cisplatin nephrotoxicity in vitro and in vivo
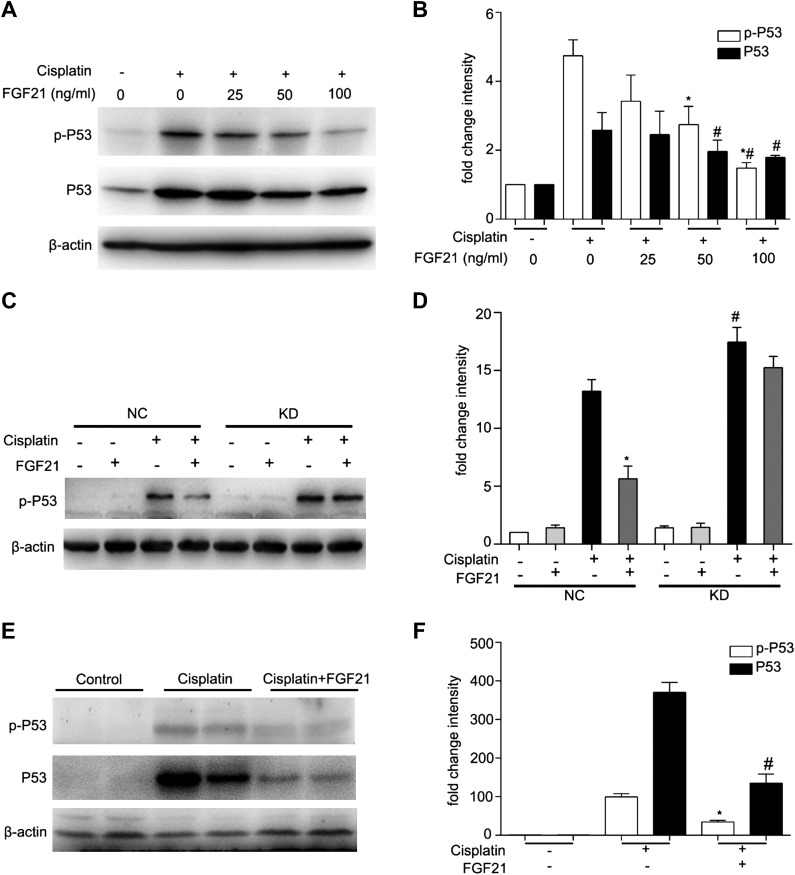
To understand how FGF21 protects cisplatin-induced AKI, we examined its effect on P53, a key player in cisplatin-induced AKI or nephrotoxicity (7, 8, 11, 12, 27). We incubated BUMPT cells with 20 μM cisplatin in the presence of different concentrations of recombinant FGF21 for 24 h and determined the expression of P53 by immunoblot analysis. Total P53 and phosphorylated P53 (p-P53) levels were increased in cisplatin-treated cells when compared with control cells, and these changes induced by cisplatin were attenuated by FGF21 in a dose-dependent manner (Fig. 7A). Quantification by densitometry further supported the suppressive effect of FGF21 on P53 (Fig. 7B). We further examined the effect of FGF21 supplementation on P53 in FGF21-knockdown cells. Supplementation of exogenous FGF21 suppressed P53 phosphorylation or activation in NC shRNA transfected cells, but the effect was largely abrogated in FGF21 KD cells (Fig. 7C). This conclusion was further supported by densitometry analysis of immunoblots of separate experiments (Fig. 7D). In vivo, cisplatin induced P53 in kidney tissues in mice, and supplementation of exogenous FGF21 suppressed P53 accumulation and phosphorylation during cisplatin treatment (Fig. 7E). Densitometry indicated that cisplatin induced dramatic increases in P53 and p-P53, which were attenuated by FGF21 (Fig. 7F). Taken together, these results suggest that FGF21 may protect kidneys in cisplatin nephrotoxicity by suppressing P53 activation.
Figure 7.
FGF21 suppresses P53 during cisplatin nephrotoxicity in vitro and in vivo. A, B) BUMPT cells were pretreated with or without recombinant FGF21 (0, 12.5, 25, 50, and 100 ng/ml) for 1 h and incubated with cisplatin (20 μM) for 24 h. Whole cell lysates were collected for immunoblot analysis of p-P53, P53, and β-actin (loading control). A) Representative immunoblots. B) Densitometry of p-P53 and P53 signals. The p-P53 and P53 signals were normalized to the β-actin signal of the same samples to determine the ratios. The ratios of the control group (in the absence of cisplatin and FGF21) were arbitrarily set as 1. Means ± sd (n = 3). *P < 0.01 vs. cisplatin-only treated cells, #P < 0.005 vs. cisplatin-only treated cells, *#P < 0.05 vs. cisplatin plus FGF21 (50 ng/ml) treated cells. C, D) BUMPT stable cell lines were pretreated with or without recombinant FGF21 (50 ng/ml) for 1 h and cultured in the presence or absence of cisplatin (20 μM) for 24 h. Whole cell lysates were collected for immunoblot analysis of p-P53 and β-actin as protein loading control. C) Representative immunoblots. D) Densitometry of p-P53 signals. The p-P53 signals in C were normalized to the β-actin signal of the same samples to determine the ratios. The ratios of the NC control group (in the absence of cisplatin and FGF21) were arbitrarily set as 1. Means ± sd (n = 3). *P < 0.001 vs. cisplatin-only treated NC group cells, #P < 0.05 vs. cisplatin-only treated NC group cells. E, F) Recombinant FGF21 (0.1 μg/g) was administered intraperitoneally to the mice (male C57BL/6, 8–10 wk) every 8 h, with the first dose 1 h before cisplatin injection. A comparable volume of PBS was given to the no-FGF21 (recombinant FGF21 was dissolved in PBS) animals. Mice were euthanized at 72 h. Kidney tissues were collected for immunoblot analysis of P53, p-P53, and β-actin (loading control). E) Representative immunoblotting. F) Densitometry of p-P53 and P53 signals. The p-P53 and P53 signals were normalized to the β-actin signal of the same samples to determine the ratios. The ratios of control group in the absence of cisplatin and FGF21 were arbitrarily set as 1. Means ± sd (n = 3). *P < 0.0005 vs. cisplatin-only treated group, #P < 0.0001 vs. cisplatin-only treated group.
DISCUSSION
The mechanisms underlying cisplatin nephrotoxicity remain largely unclear. In this study, we have shown that FGF21 is induced in kidney cells and tissues during cisplatin treatment. We have further demonstrated that the induction of FGF21 plays a protective role. In addition, we have shown that supplementation of exogenous FGF21 has beneficial effects against cisplatin-induced nephrotoxicity. Mechanistically, FGF21 can suppress P53 activity in cell and mouse models of cisplatin nephrotoxicity. Taken together, these results indicate that FGF21 is induced in renal tubular cells during cisplatin nephrotoxicity as a protective mechanism. FGF21 may protect renal tubular cells by suppressing P53.
A robust induction of FGF21 was reported recently in response to cellular and metabolic stress in liver and heart (14, 28). In kidneys, several clinical studies have shown that serum FGF21 is elevated in patients with chronic and acute renal dysfunction, including CKD or end-stage kidney disease and in patients on long-term dialysis (29–33). In general, the increase of FGF21 in circulation in patients with kidney disease is believed to be related to the decline of renal excretion of FGF21. In this study, we provided evidence that FGF21 was induced in kidney during cisplatin-induced AKI (Figs. 1C, D and 2C–E), and the induction mainly happened in the renal tubular cells (Fig. 2C). We further demonstrated that a pharmacological inhibitor of P53 (pifithrin-α) attenuated cisplatin-induced FGF21 expression in BUMPT cells (Fig. 3C), suggesting a role of P53 in FGF21 induction in cisplatin nephrotoxicity. In line with our findings, P53 was shown to mediate FGF21 transcription by binding to the promoter region of FGF21 in hepatocytes (14).
FGF21 is well known as a regulator in glucose and lipid metabolism to maintain the body’s energy metabolism balance. However, recent research has implicated FGF21 in cellular stress response under pathologic conditions (14, 17). Our current data have provided several lines of evidence that FGF21 is induced in cisplatin nephrotoxicity as a stress response to protect kidney tubule cells. First, silencing FGF21 expression sensitized BUMPT cells to cisplatin-induced cell death (Fig. 4B–D). Second, FGF21 supplementation reduced cisplatin-induced tubular cell death in vitro (Figs. 4D and 5) and in vivo (Fig. 6) and protected against renal dysfunction and tissue injury after cisplatin treatment. The antiapoptotic role of FGF21 has been demonstrated in cardiomyocytes and hepatocytes (34, 35). However, the molecular mechanism underlying the protective effect of FGF21 remains unknown. Our current data suggest that FGF21 may protect kidney tubular cells by suppressing P53.
P53-mediated tubular cell death has been demonstrated as a major mechanism of cisplatin nephrotoxicity (7, 8, 11, 12, 27). We have provided in vitro and in vivo evidence for the suppressive effect of FGF21 on P53. In BUMPT cells subjected to cisplatin treatment, FGF21 supplementation reduced total P53 and p-P53 levels in a concentration-dependent manner (Fig. 7A, C, D). Consistently, in vivo studies also demonstrated that FGF21 supplementation reduced the total P53 and p-P53 induced by cisplatin (Fig. 7B). Along with the reduction of P53 levels by FGF21, renal tubular cell death was reduced. These findings suggest that FGF21 suppresses P53-mediated tubular cell death during cisplatin-induced AKI. How FGF21 regulates P53 remains unclear and is an interesting project for future research.
In addition to acute injury, recent work has described chronic kidney pathology and CKD induced by multiple low doses of cisplatin (24, 36, 37), which represents the dosing regimen used in clinical settings for patients with cancer. Relevant to the present study, serum FGF21 has been shown to increase in patients with CKD (30). Moreover, P53 has been implicated in renal fibrosis and apoptosis in experimental models, such as the urinary obstruction model (38). Thus, it is possible FGF21 may also be induced to alleviate chronic renal pathology induced by multiple low doses of cisplatin through P53.
In the research of cisplatin-induced nephrotoxicity, it is important to consider the effect on the chemotherapy in tumors. An ideal kidney protective strategy should protect kidneys without reducing the anticancer efficacy of cisplatin (5, 24). It has been reported that the serum level and hepatocyte expression of FGF21 are elevated in patients with liver cancer, and the induction of FGF21 may involve P53 (14). Moreover, long-term administration or overexpression of FGF21 was shown to prevent chemically induced hepatocarcinogenesis in mice models (39, 40). Thus, FGF21 may not be suitable for kidney protection in patients with cancer during cisplatin chemotherapy because it may suppress the chemotherapy effect. Nonetheless, the current study has demonstrated the efficacy of induction of FGF21 in cisplatin-induced AKI or nephrotoxicity. Mechanistically, FGF21 induction under this condition is shown to be mediated by P53. Upon induction, FGF21 may suppress P53 activation during cisplatin nephrotoxicity, resulting in the protection of kidney tubular cells and consequently renal function (Fig. 8). The finding of FGF21 induction as a protective response in renal tubules by this study adds insights into the understanding of cisplatin nephrotoxicity.
Figure 8.
Diagram depicting FGF21 in cisplatin-induced renal tubular apoptosis. Upon cisplatin treatment, P53 is phosphorylated and activated to induce tubular cell apoptosis. P53 also induces FGF21 expression. Upon induction, FGF21 represses the expression and phosphorylation of P53 to reduce renal tubular apoptosis.
ACKNOWLEDGMENTS
The authors thank Dr. Jinke Cheng (Shanghai Jiao Tong University School of Medicine, Shanghai, China) for providing the FGF21 shRNA plasmids. This work was supported by the National Natural Science Foundation of China (Grants 81720108008 and 81430017), by the U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (Grants DK58831, DK87843), and by the U.S. Department of Veterans Affairs. The authors declare no conflicts of interest.
Glossary
- AKI
acute kidney injury
- BUMPT
Boston University mouse proximal tubular
- BUN
blood urea nitrogen
- C-caspase 3
cleaved caspase-3
- CKD
chronic kidney disease
- FGF
fibroblast growth factor
- KD
knockdown
- NC
negative control
- p-P53
phosphorylated P53
- shRNA
short hairpin RNA
AUTHOR CONTRIBUTIONS
F. Li and Z. Dong designed the research; F. Li and Z. Liu performed the experiments; F. Li, C. Tang, J. Cai, and Z. Dong analyzed the data; F. Li drafted the paper; and F. Li, C. Tang, J. Cai, and Z. Dong revised the paper for publication.
REFERENCES
- 1.Agarwal A., Dong Z., Harris R., Murray P., Parikh S. M., Rosner M. H., Kellum J. A., Ronco C.; Acute Dialysis Quality Initiative XIII Working Group . (2016) Cellular and molecular mechanisms of AKI. J. Am. Soc. Nephrol. 27, 1288–1299 10.1681/ASN.2015070740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linkermann A., Chen G., Dong G., Kunzendorf U., Krautwald S., Dong Z. (2014) Regulated cell death in AKI. J. Am. Soc. Nephrol. 25, 2689–2701 10.1681/ASN.2014030262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basile D. P., Bonventre J. V., Mehta R., Nangaku M., Unwin R., Rosner M. H., Kellum J. A., Ronco C.; ADQI XIII Work Group . (2016) Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J. Am. Soc. Nephrol. 27, 687–697 10.1681/ASN.2015030309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Lippard S. J. (2005) Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 4, 307–320 10.1038/nrd1691 [DOI] [PubMed] [Google Scholar]
- 5.Pabla N., Dong Z. (2008) Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 73, 994–1007 10.1038/sj.ki.5002786 [DOI] [PubMed] [Google Scholar]
- 6.Miller R. P., Tadagavadi R. K., Ramesh G., Reeves W. B. (2010) Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2, 2490–2518 10.3390/toxins2112490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang M., Dong Z. (2008) Regulation and pathological role of p53 in cisplatin nephrotoxicity. J. Pharmacol. Exp. Ther. 327, 300–307 10.1124/jpet.108.139162 [DOI] [PubMed] [Google Scholar]
- 8.Yan M., Tang C., Ma Z., Huang S., Dong Z. (2016) DNA damage response in nephrotoxic and ischemic kidney injury. Toxicol. Appl. Pharmacol. 313, 104–108 10.1016/j.taap.2016.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang M., Wei Q., Wang J., Du Q., Yu J., Zhang L., Dong Z. (2006) Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene 25, 4056–4066 10.1038/sj.onc.1209440 [DOI] [PubMed] [Google Scholar]
- 10.Seth R., Yang C., Kaushal V., Shah S. V., Kaushal G. P. (2005) p53-dependent caspase-2 activation in mitochondrial release of apoptosis-inducing factor and its role in renal tubular epithelial cell injury. J. Biol. Chem. 280, 31230–31239 10.1074/jbc.M503305200 [DOI] [PubMed] [Google Scholar]
- 11.Wei Q., Dong G., Yang T., Megyesi J., Price P. M., Dong Z. (2007) Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am. J. Physiol. Renal Physiol. 293, F1282–F1291 10.1152/ajprenal.00230.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang D., Liu Y., Wei Q., Huo Y., Liu K., Liu F., Dong Z. (2014) Tubular p53 regulates multiple genes to mediate AKI. J. Am. Soc. Nephrol. 25, 2278–2289 10.1681/ASN.2013080902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher F. M., Maratos-Flier E. (2016) Understanding the physiology of FGF21. Annu. Rev. Physiol. 78, 223–241 10.1146/annurev-physiol-021115-105339 [DOI] [PubMed] [Google Scholar]
- 14.Yang C., Lu W., Lin T., You P., Ye M., Huang Y., Jiang X., Wang C., Wang F., Lee M. H., Yeung S. C., Johnson R. L., Wei C., Tsai R. Y., Frazier M. L., McKeehan W. L., Luo Y. (2013) Activation of liver FGF21 in hepatocarcinogenesis and during hepatic stress. BMC Gastroenterol. 13, 67 10.1186/1471-230X-13-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams A. C., Kharitonenkov A. (2012) FGF21: the center of a transcriptional nexus in metabolic regulation. Curr. Diabetes Rev. 8, 285–293 10.2174/157339912800840505 [DOI] [PubMed] [Google Scholar]
- 16.Laeger T., Henagan T. M., Albarado D. C., Redman L. M., Bray G. A., Noland R. C., Münzberg H., Hutson S. M., Gettys T. W., Schwartz M. W., Morrison C. D. (2014) FGF21 is an endocrine signal of protein restriction. J. Clin. Invest. 124, 3913–3922 10.1172/JCI74915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feingold K. R., Grunfeld C., Heuer J. G., Gupta A., Cramer M., Zhang T., Shigenaga J. K., Patzek S. M., Chan Z. W., Moser A., Bina H., Kharitonenkov A. (2012) FGF21 is increased by inflammatory stimuli and protects leptin-deficient ob/ob mice from the toxicity of sepsis. Endocrinology 153, 2689–2700 10.1210/en.2011-1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye D., Wang Y., Li H., Jia W., Man K., Lo C. M., Wang Y., Lam K. S., Xu A. (2014) Fibroblast growth factor 21 protects against acetaminophen-induced hepatotoxicity by potentiating peroxisome proliferator-activated receptor coactivator protein-1α-mediated antioxidant capacity in mice. Hepatology 60, 977–989 10.1002/hep.27060 [DOI] [PubMed] [Google Scholar]
- 19.Wente W., Efanov A. M., Brenner M., Kharitonenkov A., Köster A., Sandusky G. E., Sewing S., Treinies I., Zitzer H., Gromada J. (2006) Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes 55, 2470–2478 10.2337/db05-1435 [DOI] [PubMed] [Google Scholar]
- 20.Kim H. W., Lee J. E., Cha J. J., Hyun Y. Y., Kim J. E., Lee M. H., Song H. K., Nam D. H., Han J. Y., Han S. Y., Han K. H., Kang Y. S., Cha D. R. (2013) Fibroblast growth factor 21 improves insulin resistance and ameliorates renal injury in db/db mice. Endocrinology 154, 3366–3376 10.1210/en.2012-2276 [DOI] [PubMed] [Google Scholar]
- 21.Zhang C., Shao M., Yang H., Chen L., Yu L., Cong W., Tian H., Zhang F., Cheng P., Jin L., Tan Y., Li X., Cai L., Lu X. (2013) Attenuation of hyperlipidemia- and diabetes-induced early-stage apoptosis and late-stage renal dysfunction via administration of fibroblast growth factor-21 is associated with suppression of renal inflammation. PLoS One 8, e82275 10.1371/journal.pone.0082275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rusu C. C., Racasan S., Kacso I. M., Moldovan D., Potra A., Tirinescu D., Budurea C., Orasan R., Patiu I. M., Bondor C. I., Vladutiu D., Caprioara M. G. (2017) The metabolic hormone FGF21 is associated with endothelial dysfunction in hemodialysis patients. Int. Urol. Nephrol. 49, 517–523 10.1007/s11255-016-1474-x [DOI] [PubMed] [Google Scholar]
- 23.Bhatt K., Zhou L., Mi Q. S., Huang S., She J. X., Dong Z. (2010) MicroRNA-34a is induced via p53 during cisplatin nephrotoxicity and contributes to cell survival. Mol. Med. 16, 409–416 10.2119/molmed.2010-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pabla N., Dong G., Jiang M., Huang S., Kumar M. V., Messing R. O., Dong Z. (2011) Inhibition of PKCδ reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J. Clin. Invest. 121, 2709–2722 10.1172/JCI45586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livingston M. J., Ding H. F., Huang S., Hill J. A., Yin X. M., Dong Z. (2016) Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy 12, 976–998 10.1080/15548627.2016.1166317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang M., Yi X., Hsu S., Wang C. Y., Dong Z. (2004) Role of p53 in cisplatin-induced tubular cell apoptosis: dependence on p53 transcriptional activity. Am. J. Physiol. Renal Physiol. 287, F1140–F1147 10.1152/ajprenal.00262.2004 [DOI] [PubMed] [Google Scholar]
- 27.Hao J., Wei Q., Mei S., Li L., Su Y., Mei C., Dong Z. (2017) Induction of microRNA-17-5p by p53 protects against renal ischemia-reperfusion injury by targeting death receptor 6. Kidney Int. 91, 106–118 10.1016/j.kint.2016.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Planavila A., Redondo I., Hondares E., Vinciguerra M., Munts C., Iglesias R., Gabrielli L. A., Sitges M., Giralt M., van Bilsen M., Villarroya F. (2013) Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat. Commun. 4, 2019 10.1038/ncomms3019 [DOI] [PubMed] [Google Scholar]
- 29.Loeffler I., Hopfer U., Koczan D., Wolf G. (2011) Type VIII collagen modulates TGF-β1-induced proliferation of mesangial cells. J. Am. Soc. Nephrol. 22, 649–663 10.1681/ASN.2010010098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hindricks J., Ebert T., Bachmann A., Kralisch S., Lössner U., Kratzsch J., Stolzenburg J. U., Dietel A., Beige J., Anders M., Bast I., Blüher M., Stumvoll M., Fasshauer M. (2014) Serum levels of fibroblast growth factor-21 are increased in chronic and acute renal dysfunction. Clin. Endocrinol. (Oxf.) 80, 918–924 10.1111/cen.12380 [DOI] [PubMed] [Google Scholar]
- 31.Lin Z., Zhou Z., Liu Y., Gong Q., Yan X., Xiao J., Wang X., Lin S., Feng W., Li X. (2011) Circulating FGF21 levels are progressively increased from the early to end stages of chronic kidney diseases and are associated with renal function in Chinese. PLoS One 6, e18398 10.1371/journal.pone.0018398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein S., Bachmann A., Lössner U., Kratzsch J., Blüher M., Stumvoll M., Fasshauer M. (2009) Serum levels of the adipokine FGF21 depend on renal function. Diabetes Care 32, 126–128 10.2337/dc08-1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han S. H., Choi S. H., Cho B. J., Lee Y., Lim S., Park Y. J., Moon M. K., Lee H. K., Kang S. W., Han D. S., Kim Y. B., Jang H. C., Park K. S. (2010) Serum fibroblast growth factor-21 concentration is associated with residual renal function and insulin resistance in end-stage renal disease patients receiving long-term peritoneal dialysis. Metabolism 59, 1656–1662 10.1016/j.metabol.2010.03.018 [DOI] [PubMed] [Google Scholar]
- 34.Yu Y., Bai F., Liu Y., Yang Y., Yuan Q., Zou D., Qu S., Tian G., Song L., Zhang T., Li S., Liu Y., Wang W., Ren G., Li D. (2015) Fibroblast growth factor (FGF21) protects mouse liver against D-galactose-induced oxidative stress and apoptosis via activating Nrf2 and PI3K/Akt pathways. Mol. Cell. Biochem. 403, 287–299 10.1007/s11010-015-2358-6 [DOI] [PubMed] [Google Scholar]
- 35.Zhang C., Huang Z., Gu J., Yan X., Lu X., Zhou S., Wang S., Shao M., Zhang F., Cheng P., Feng W., Tan Y., Li X. (2015) Fibroblast growth factor 21 protects the heart from apoptosis in a diabetic mouse model via extracellular signal-regulated kinase 1/2-dependent signalling pathway. Diabetologia 58, 1937–1948 10.1007/s00125-015-3630-8 [DOI] [PubMed] [Google Scholar]
- 36.Sharp C. N., Doll M. A., Dupre T. V., Shah P. P., Subathra M., Siow D., Arteel G. E., Megyesi J., Beverly L. J., Siskind L. J. (2016) Repeated administration of low-dose cisplatin in mice induces fibrosis. Am. J. Physiol. Renal Physiol. 310, F560–F568 10.1152/ajprenal.00512.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres R., Velazquez H., Chang J. J., Levene M. J., Moeckel G., Desir G. V., Safirstein R. (2016) Three-dimensional morphology by multiphoton microscopy with clearing in a model of cisplatin-induced CKD. J. Am. Soc. Nephrol. 27, 1102–1112 10.1681/ASN.2015010079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J., Wang J., Li H., Wang S., Xiang X., Zhang D. (2016) p53 activates miR-192-5p to mediate vancomycin induced AKI. Sci. Rep. 6, 38868 10.1038/srep38868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang X., Yu C., Jin C., Yang C., Xie R., Cao D., Wang F., McKeehan W. L. (2006) Forced expression of hepatocyte-specific fibroblast growth factor 21 delays initiation of chemically induced hepatocarcinogenesis. Mol. Carcinog. 45, 934–942 10.1002/mc.20241 [DOI] [PubMed] [Google Scholar]
- 40.Xu P., Zhang Y., Wang W., Yuan Q., Liu Z., Rasoul L. M., Wu Q., Liu M., Ye X., Li D., Ren G. (2015) Long-term administration of fibroblast growth factor 21 prevents chemically-induced hepatocarcinogenesis in mice. Dig. Dis. Sci. 60, 3032–3043 10.1007/s10620-015-3711-z [DOI] [PubMed] [Google Scholar]