Abstract
Sphingosine-1-phosphate (S1P) plays important roles in cardiovascular development and immunity. S1P is abundant in plasma because erythrocytes—the major source of S1P—lack any S1P-degrading activity; however, much remains unclear about the source of the plasma S1P precursor, sphingosine (SPH), derived mainly from the hydrolysis of ceramides by the action of ceramidases that are encoded by 5 distinct genes, acid ceramidase 1 (ASAH1)/Asah1, ASAH2/Asah2, alkaline ceramidase 1 (ACER1)/Acer1, ACER2/Acer2, and ACER3/Acer3, in humans/mice. Previous studies have reported that knocking out Asah1 or Asah2 failed to reduce plasma SPH and S1P levels in mice. In this study, we show that knocking out Acer1 or Acer3 also failed to reduce the blood levels of SPH or S1P in mice. In contrast, knocking out Acer2 from either whole-body or the hematopoietic lineage markedly decreased the blood levels of SPH and S1P in mice. Of interest, knocking out Acer2 from whole-body or the hematopoietic lineage also markedly decreased the levels of dihydrosphingosine (dhSPH) and dihydrosphingosine-1-phosphate (dhS1P) in blood. Taken together, these results suggest that ACER2 plays a key role in the maintenance of high plasma levels of sphingoid base-1-phosphates—S1P and dhS1P—by controlling the generation of sphingoid bases—SPH and dhSPH—in hematopoietic cells.—Li, F., Xu, R., Low, B. E., Lin, C.-L., Garcia-Barros, M., Schrandt, J., Mileva, I., Snider, A., Luo, C. K., Jiang, X.-C., Li, M.-S., Hannun, Y. A., Obeid, L. M., Wiles, M. V., Mao, C. Alkaline ceramidase 2 is essential for the homeostasis of plasma sphingoid bases and their phosphates.
Keywords: sphingosine-1-phosphate, sphingolipid, plasma, hematopoietic cell, erythrocyte
Sphingosine-1-phosphate (S1P) is a bioactive lipid that mediates various biologic processes by binding 1 or more of its 5 GPCRs, designated S1P1–5 (1–4), or through intracellular targets (5–7). S1P is abundant in mouse (8) and human blood (9), but its levels are low in most peripheral tissues, including lymphoid tissues in mice (10). S1P concentration gradients formed between blood and lymphoid organs—the thymus, spleen, and lymph nodes—are required for the egress of lymphocytes from lymphoid organs to the blood; hence, the perturbation of such gradients may lead to peripheral lymphopenia (11–15). Plasma S1P and its receptors are also essential for vascular development and homeostasis—for example, the depletion of plasma S1P by blocking its generation (16, 17) or knocking out its receptor, S1P1 (18), impairs the maturation of blood vessels in mouse embryos, which leads to embryonic death. Apart from these physiologic roles, an aberrant increase in blood S1P levels has been implicated in the pathogenesis of several inflammatory diseases and cardiovascular diseases (3). Apart from S1P, dihydrosphingosine-1-phosphate (dhS1P), a saturated analog of S1P, is also much more abundant in plasma than in the peripheral tissues in humans and mice, although its levels are several-fold lower than those of S1P in plasma (19). Although its biologic functions have not been investigated as much as S1P, limited studies have indicated that dhS1P may play roles that are similar to S1P in certain biologic processes, such as cell survival (20), while exerting its distinct biologic functions (21–23).
Considering the critical physiologic and pathophysiologic roles of S1P and dhS1P—collectively called sphingoid base-1-phosphates (SBPs)—it is crucial to understand their metabolism. S1P and dhS1P are formed by the phosphorylation of sphingosine (SPH) and dihydrosphingosine (dhSPH), respectively, by the action of the sphingosine kinases, SPHK1 (24) and SPHK2 (25), in mammalian cells (Supplemental Fig. 1). Once formed, (dh)S1Ps are cleaved into phosphoethanolamine and hexadecenal/hexadecanal by the action of S1P lyase encoded by the SGPL1 gene (26). SGPL1 is deficient in erythrocytes and platelets, and SBPs thus accumulate to high levels in these anucleated cells (8). Because erythrocytes are the most abundant hematopoietic cells that release SBPs constitutively, they are the major source of plasma SBPs (8, 27); however, it is unclear where and how the plasma (dh)S1P precursors, (dh)SPHs, are generated.
SPH is only generated from the hydrolysis of ceramides by the action of ceramidases, which are classified into the acid, neutral, and alkaline ceramidase subtypes in mouse or human cells (28, 29), whereas dhSPH can be generated via the anabolic pathway that begins with the condensation of serine and palmitoyl-CoA or the hydrolysis of certain dihydroceramide species by the action of members in the alkaline ceramidase family (Supplemental Fig. 1). Patients who are deficient in the acid ceramidase gene (ASAH1) have plasma SPH and S1P levels that are similar to those of healthy individuals (30), and deficiency in the acid ceramidase gene (Asah1) does not affect blood SPH and S1P levels in mice (30). Mice that are deficient in the neutral ceramidase gene (Asah2) have plasma S1P levels that are similar to those of WT mice (31). In contrast to Asah1 or Asah2 inactivation, we have previously demonstrated that the treatment of mice with an alkaline ceramidase inhibitor markedly reduces blood SPH and S1P levels (32). These results suggest that 1 or more of the alkaline, but not acid or neutral ceramidase subtypes, may play an important role in the formation of the plasma S1P precursor, SPH, in mice, although it remains unclear which specific alkaline ceramidases are involved. Of interest, no studies have addressed the role of ceramidases in the regulation of plasma dhS1P.
In this study, we demonstrate that knocking out Acer2 markedly reduces the levels of SPH, dhSPH, S1P, and dhS1P in whole blood, plasma, erythrocytes, and platelets in mice, whereas knocking out Acer1 or Acer3 fails to do so, which suggests that, among the known ceramidases, only alkaline ceramidase 2 (ACER2) plays a major role in maintaining high blood S1P and dhS1P levels via the generation of SPH and dhSPH, respectively, in hematopoietic cells. As Acer2 deficiency does not seem to result in major pathologies in mice, at least at young ages, ACER2 may serve as an effective, but safe, target for interventions in (dh)S1P-associated inflammatory, cardiovascular, and other diseases.
MATERIALS AND METHODS
Animals
A mouse strain that is deficient in Acer1 (33) or Acer3 (34) was generated previously in our laboratory. To generate an Acer2 knockout mouse strain, female C57BL/6J mice were superovulated and mated, and zygotes were collected and microinjected with ∼2–5 pl of Cas9 mRNA (100 ng/µl) and 2 single-guide RNAs (sgRNAs; 50 ng/µl each) as previously described (35, 36). Cas9 mRNA was synthesized from the DNA template—the Cas9 coding sequence preceded with T7 promoter—by using mMESSAGE mMCHINE T7 ULTRA Transcription Kit (Ambion, Austin, TX, USA) according to manufacturer instructions. sgRNAs were designed (according to http://crispr.mit.edu) to flank exon 2 of the Acer2 gene (target sites: 5′-GACGTTGCTGATCTGGAG-3′, 5′-GTGGGAACGGGACTGAA-3′). Of 18 offspring, at least 4 founders demonstrated a dropout deletion of exon 2 as detected by PCR and sequencing. Founders were backcrossed to wild-type (WT) C57BL/6J mice, and N1 heterozygotes (Acer2+/−) with a 452-bp deletion allele—removing all of exon 2—were selected to establish a new Acer2 mutant C57BL/6J strain, designated C57BL/6J-Acer2 < em1Mvw > /MvwJ (JAX stock 26793; The Jackson Laboratory) and abbreviated here to Acer2−/− mice. To generate Acer1−/−Acer2−/− or Acer2−/−Acer3−/− double-knockout mice, Acer1−/− or Acer3−/− single-knockout mice were crossed with Acer2−/− single-knockout mice. The resultant heterozygous offspring, Acer1+/−Acer2+/− or Acer2+/−Acer3+/−, were then intercrossed to produce Acer1−/−Acer2−/− or Acer2−/−Acer3−/− mice, respectively.
All mice used in this study were housed under the conventional laboratory conditions of a constant room temperature (22°C), humidity level (55%), and 12-h light/dark photoperiod, with food (mouse chow; W.F. Fisher & Son, Somerville, NJ, USA) and water available ad libitum. Both male and female mice were used for all experiments and were equally distributed within experimental and control groups. All animal studies were performed according to procedures approved by the Institutional Animal Care and Use Committee at Stony Brook University (Stony Brook, NY, USA).
Genotyping of animals
To genotype Acer2 mutant mice, tail tip (∼2-mm) biopsies were digested for 30 min at 95°C in alkaline lysis buffer that contained 25 mM NaOH and 0.2 mM EDTA, and tissues were homogenized by stirring with a pipet tip before being neutralized with 40 mM Tris-HCl buffer (pH 7.4). Tissue homogenates were diluted 5 times with d2H2O, and 1 μl of the diluted tissue homogenate from each sample was combined with 1 μl of the genotyping primer pair (5′-CGCGGTCTTTACGATGTTCTA-3′ and 5′-CTCTGAACCTCCACGATAACAG-3′), 8 μl H2O, and 10 μl HotStarTag Plus Master Mix (Qiagen, Germantown, MD, USA). PCR mixtures were subjected to 30 cycles of denaturing at 95°C, annealing at 58°C, and extension at 72°C, and amplicons of 796 and 344 bp for WT and deletion products, respectively, were analyzed by agarose gel electrophoresis (Supplemental Fig. 2A). Acer1 and Acer3 mutant mice were genotyped as described in Lin et al. (33) and Truett et al. (37), respectively.
To genotype bone marrow chimeric mice, blood samples (65–100 μl/mouse) were collected into an EDTA-coated tube and mixed by vortex with 200 µl lysis buffer and 10 mM Tris-HCl (pH 7.5) that contained 0.32 M sucrose, 5 mM MgCl2, and 1% v/v Triton X-100. The tube was then centrifuged at 16,000 g for 25 s to pellet nuclei, which were subjected to PCR analysis as previously described (Supplemental Fig. 2B).
Bone marrow transplantation
Autologous bone marrow transplantation was performed as described previously (38). In brief, recipient mice at age 6 wk were treated with 1.1 mg/ml neomycin in drinking water for 1 d before being exposed to γ-irradiation (900 rad at a rate of ∼80 rad/min) from a 137Cs source in a Gamma 40 Exactor (Commercial Products; Atomic Energy of Canada, Chalk River, ON, Canada). Total bone marrow cells were isolated from donor mice by flushing the femur and tibia from both legs twice with 1 ml HBSS. Bone marrow cells (5 × 105) in 150–200 μl were injected intraveniously into each of the lethally irradiated recipient mice 16 h after radiation. Genotypes of hematopoietic cells in the recipient mice were confirmed by PCR as previously described (Supplemental Fig. 1B).
Quantitative PCR
RNAs were extracted from mouse embryos at embryonic development d 9.5 and reverse-transcribed into cDNAs (34) before being subjected to quantitative PCR analysis using the primer pairs that are specific for Acer2 (5′-GTGTGGCATATTCTCATCTG-3′/5′-TAAGGGACACCAATAAAAGC-3′) or β-actin (5′-GATGTATGAAGGCTTTGGTC-3′/5′-TGTGCACTTTTATTGGTCTC-3′). Quantitative PCR was performed on an ABI Prism 7000 system (Applied Biosystems, Foster City, CA, USA) and analyzed by using Q-Gene software, which expresses data as mean normalized expression (39). Mean normalized expression is directly proportional to the mRNA levels of a target gene (Acer2) relative to those of the reference gene (β-actin).
Liquid chromatography–tandem mass spectrometry sphingolipid analysis
Whole blood was collected into EDTA-coated tubes via cardiac puncture from mice after deep terminal anesthesia, and plasma, erythrocytes, or platelets were prepared from whole blood samples as previously described (32). Lung and colon tissues were dissected from euthanized animals and homogenized in ice-cold 25 mM Tris-HCl buffer (pH 7.4) that contained 150 mM NaCl as previously described (34). Whole blood, plasma, erythrocytes, platelets, and tissue homogenates were subjected to liquid chromatography–tandem mass spectrometry (LC-MS/MS) analyses at the Lipidomics Core Facility at Stony Brook University for various sphingolipids as described in our previous study (34). In brief, 50 μl of an internal sphingolipid standard mixture (IS) at a concentration of 1 μM was added to each cell pellet or tissue homogenate. When C18-sphingoid bases and their derivatives were measured, 3 types of IS mixtures were used: 1) a mixture of C17SPH, C17SPH-1-phosphate, C17-dihydrosphingosine, 13C16-ceramide (d13:1/16), 17C16-ceramide (d17:1/16), C17-ceramide (d18:1/17:0) (32), and 17C24:1-ceramide (d17:1/24:1); 2) a mixture of C8-galactosyl-ceramide and C12-glucosylceramide; and 3) a mixture of C6-sphingomylin, C12-sphingomyelin, C12-dihydrosphingomyelin, and 17C-sphingomyelin. When C16SPH, C16dhSPH, C17SPH, C17dhSPH, C20SPH, C20dhSPH, and their phosphates were measured, a mixture of 13C16-ceramide and 13C22-ceramide was used as IS. Lipids were extracted by using 4 ml of the ethyl acetate/isopropanol/water [60/28/12% (v/v)] solvent system. After centrifugation, a 0.5-ml aliquot of the lipid extract from each sample was used to determine total phospholipids and the remaining was used for LC-MS/MS. Lipid extracts from each sample were dried under a stream of nitrogen gas, dissolved in 150 μl of mobile phase (methanol with 0.2% formic acid and 1 mM ammonium formate), and injected on the TSQ Quantum Ultra LC-MS/MS system. Lipids were gradient eluted from the Spectra C8SR column (150 × 3.0 mm, 3-f particle size), with a mobile phase system that consisted of methanol acidified with 0.2% formic acid and 1.0 mM ammonium formate. Peaks that corresponded to the target analytes and IS were identified and processed using LCQuan software (Thermo Fisher Scientific, Waltham, MA, USA). Quantitative analyses of endogenous sphingolipids were based on calibration curves that were generated by standards purchased from Avanti Polar Lipids (Alabaster, AL, USA). For the analytes for which there was not a commercially available standard, the closest by mass and structure standard was used for quantification. Target analyte/IS peak area ratios were compared with the calibration curves by using a linear regression model. Levels of a particular sphingolipid in cells or peripheral tissues were normalized to inorganic phosphate released from total phospholipids or total proteins, expressed as sphingolipid (picomoles per micromole inorganic phosphate) and [picomoles per milligram protein (Pr)], respectively. We confirmed that C17SPH, C17dhSPH, and their phosphates were undetectable in mouse blood and lung tissues, and these lipids were thus appropriate ISs for the LC-MS/MS analyses of sphingolipids in mouse tissue.
Protein extraction and Western blot analysis
Mouse embryos at embryonic development d 9.5 were subjected to protein extraction and Western blot analyses, as described in our previous study (34), with the primary Ab against ACER2 (1:2000) or GM130 (1:1000; Cell Signaling Technology, Beverly, MA, USA), followed by horseradish peroxidase–conjugated secondary Ab. Protein bands were detected with an ECL kit (Thermo Fisher Scientific).
Complete blood count analysis
Blood samples were collected into EDTA-coated microcentrifuge tubes (Becton Dickinson, Franklin Lakes, NJ, USA) via superficial temporal vein phlebotomy. Whole blood samples were analyzed in duplicate for complete blood count (CBC) on a Hemavet 950FS Multi Species Hematology System (Drew Scientific, Waterbury, CT, USA) that was programmed with mouse hematology settings. Sample processing and system maintenance were performed as described in the manufacturer’s operating instructions.
Fluorescence-activated cell sorting analysis
Lymphocytes in blood were analyzed by flow cytometry using FACSCaliber (Becton Dickinson) as previously described (10). In brief, blood samples were collected into EDTA-coated microcentrifuge tubes, and a 50-μl blood sample from each mouse was incubated for 20 min at room temperature with anti-CD19 Ab conjugated with the fluorophore, PE-CY7, or a mixture of anti-CD3ε, CD4, and CD8 Abs conjugated with the fluorophores, PE-CY7, PE, and FITC, respectively. Ab-labeled blood cell samples were treated with Gey’s hemolytic solution to lyse erythrocytes before the addition of countBright absolute counting beads (Thermo Fisher Scientific). Finally, flow cytometry was performed to analyze CD3+CD4+, CD3+CD8+, or C19+ cells.
Statistical analysis
Data are presented as means ± sd and compared by 2-tailed Student’s t tests using Prism 6 (GraphPad Prism, La Jolla, CA, USA). A difference between 2 groups or treatments was considered significant at a value of P < 0.05.
RESULTS
Knockout of Acer1 or Acer3 does not reduce blood S1P levels in mice
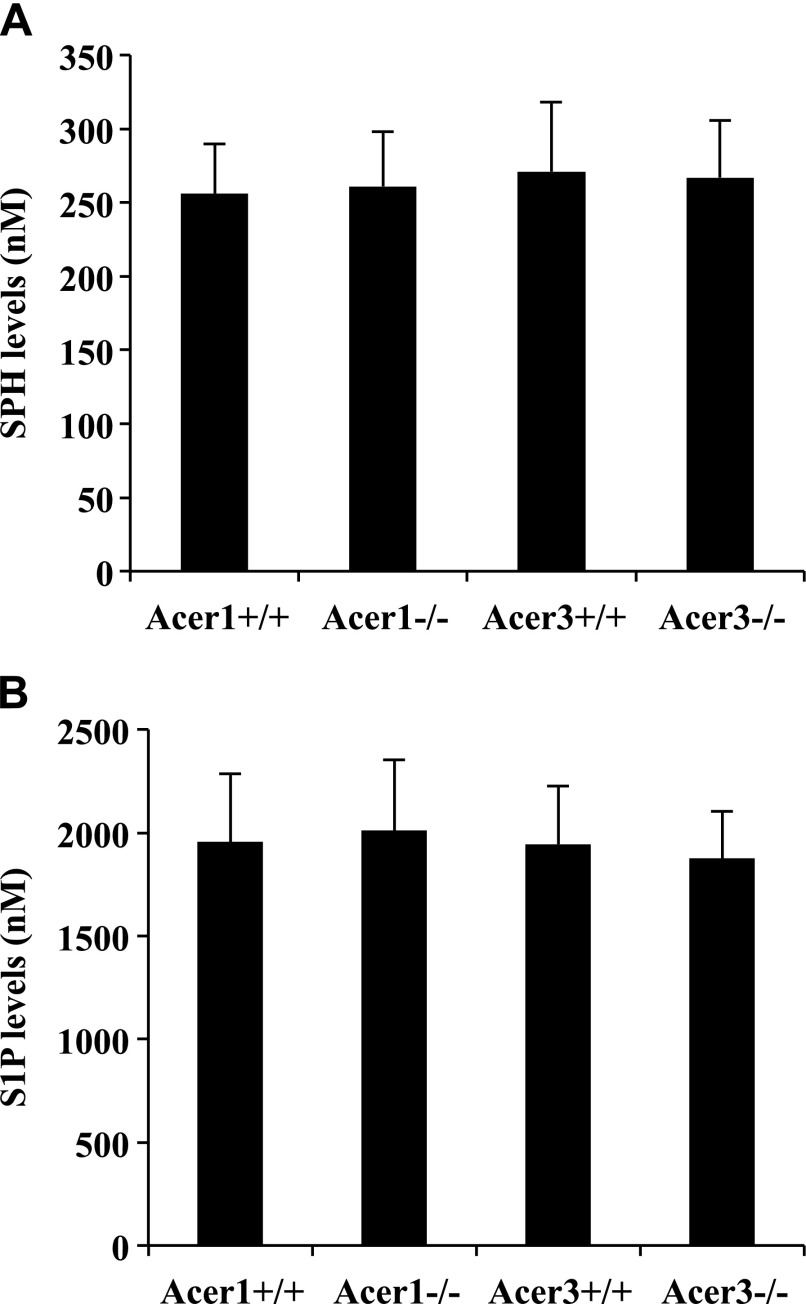
Our previous study indicated that alkaline ceramidase activity is important for the generation of both SPH and S1P in plasma, because treatment with the alkaline ceramidase inhibitor, D-MAPP [(1S,2R-D-erythro-2-N-myristoylamino)-1-phenyl-1-propanol], markedly reduces the levels of both SPH and S1P in mouse blood and plasma (32). As there are 3 mouse alkaline ceramidase genes (Acer1, Acer2, and Acer3) (28, 29), we defined their role in controlling circulating S1P levels by using mutant mice in which alkaline ceramidase genes were individually knocked out. Because the Acer1 (33) and Acer3 (34) knockout mouse lines have been established in our laboratory before this study, we first measured SPH and S1P levels in whole blood in these knockout mice and their WT littermates. We demonstrated that knocking out neither Acer1, nor Acer3, decreased the levels of SPH (Fig. 1A) and S1P (Fig. 1B) in mouse blood. These results suggest that Acer1 and Acer3 play limited roles in controlling blood S1P levels.
Figure 1.
Knocking out Acer1 or Acer3 does not reduce the blood levels of SPH and S1P in mice. Whole blood was collected from Acer1−/− mice and their Acer1+/+ littermates or from Acer3−/− mice and their Acer3+/+ littermates at age 6 wk and was subjected to LC-MS/MS analyses for SPH (A) or S1P (B). Data represent means ± sd (n = 3 mice/genotype).
Generation of Acer2 knockout mice
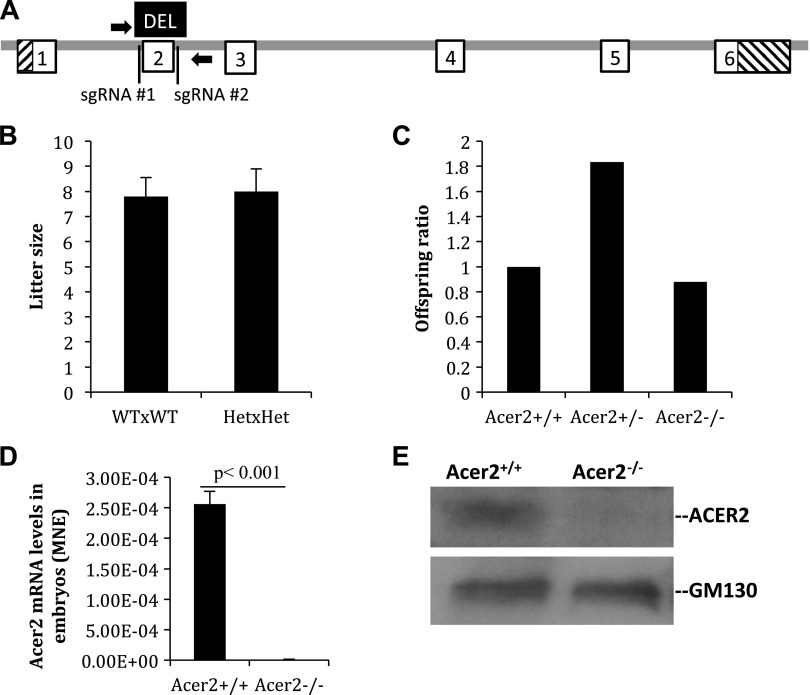
To determine whether Acer2 has a role in the regulation of blood S1P and dhS1P levels, we generated Acer2 mutant mouse strains by using CRISPR/Cas9 technology (36, 40, 41) as depicted in Fig. 2A. The Acer2 gene consists of 6 exons and 5 introns, and we chose to delete exon 2 as it encodes a highly conserved protein segment among members in the alkaline ceramidase family. Founder mice that carry the exon 2 deletion allele of the Acer2 gene were generated from zygotes that were injected with Cas9 mRNA and 2 CRISPR guide RNAs (sgRNA#1 and sgRNA#2) that flank and target exon 2 of the Acer2 gene. Founder mice were backcrossed to WT C57BL/6J mice to generate Acer2+/− mice that were heterozygous for the exon 2 deletion of the Acer2 gene. Intercross of Acer2+/− mice produced the same number of live-born neonates per litter as the intercross of Acer2+/+ mice (Fig. 2B). Homozygous mice—C57BL/6J-Acer2〈em1Mvw〉/MvwJ, abbreviated here as Acer2−/− mice—were weaned at the expected Mendelian ratio (Fig. 2C) and demonstrated no overt abnormalities, at least during the first 6 mo of age (data not shown). PCR-based genotyping indicated that Acer2+/− mice contained 1 WT allele and 1 exon 2 deletion allele, and Acer2−/− mice 2 exon 2 deletion alleles (Supplemental Fig. 2A). To confirm whether the deletion of exon 2 inactivates the Acer2 gene, we determined the expression of Acer2 mRNA and protein in Acer2+/+ or Acer2−/− embryos. Quantitative PCR analysis demonstrated that Acer2 mRNA levels were detected at high levels in Acer2+/+, but not Acer2−/− embryo (Fig. 2D). Western blot analysis detected ACER2 protein in Acer2+/+, but not Acer2−/− embryos (Fig. 2E). These results suggest that Acer2+/− females are fertile and that Acer2 deficiency in the fetus does not seem to affect fetal development and survival.
Figure 2.
Generation of Acer2 knockout mice. A) Schematic of the CRISPR/Cas9 system-mediated inactivation of the Acer2 gene. The mouse Acer2 gene consists of 5 introns (gray) and 6 exons (boxed) that encode both coding sequence (white) and noncoding sequence (hashed). In the C57BL/6J-Acer2〈em1Mvw〉/MvwJ (Acer2−/−) strain allele, a fragment (black) that encompasses the whole exon 2 was deleted by using Cas9 and sgRNA#1 and sgRNA#2. Mice were genotyped with PCR primers (arrows) as indicated. B) Acer2+/− and Acer2+/+ females were crossed with Acer2+/− and Acer2+/+ males, respectively. Live-born neonates were numerated and the average litter size was computed. Data represent means ± sd. C) Acer2+/− mice (3 pairs) were intercrossed to produce pups (3 litters/mating pair) that were weaned and genotyped at 21 d postbirth. Total numbers of WT (Acer2+/+), heterozygous (Acer2+/−), and homozygous (Acer2−/−) offspring were recorded, and the ratio of the offspring of 3 genotypes was computed. Data represent means ± sd. D) Quantitative PCR analysis for Acer2 mRNA levels in embryonic development d 9 Acer2+/+ or Acer2−/− embryos. E) Western blot analysis for ACER2 protein levels in embryonic development d 9 Acer2+/+ or Acer2−/− mice. MNE, mean normalized expression.
Knocking out Acer2 globally reduces the blood levels of sphingoid bases and their phosphates in mice
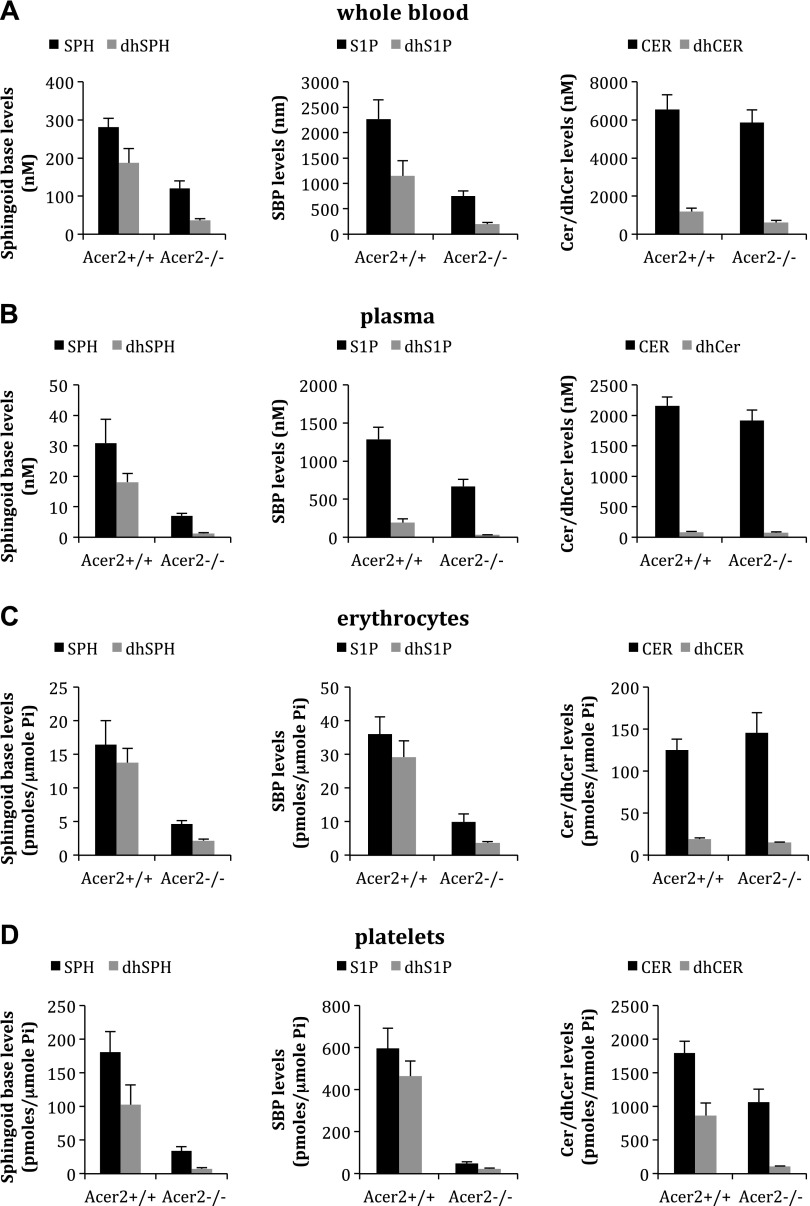
To determine whether Acer2 knockout reduces circulating (dh)SPH and (dh)S1P, we first measured the levels of these sphingolipids in the circulation from Acer2−/− and Acer2+/+ mice. LC-MS/MS revealed that knocking out Acer2 markedly reduced the levels of (dh)S1P and (dh)SPH in whole blood (Fig. 3A), plasma (Fig. 3B), erythrocytes (Fig. 3C), and platelets (Fig. 3D) in mice. Knocking out Acer2 did not affect the levels of ceramides (Fig. 3A), sphingomyelins (Supplemental Fig. 3C), or monohexosylceramides (Supplemental Fig. 3D) in whole blood. Of interest, we additionally revealed that knocking out Acer2 markedly decreased the levels of ceramides (Fig. 3D and Supplemental Fig. 3A) and dihydroceramides (Fig. 3D and Supplemental Fig. 3B) in platelets. These results suggest that ACER2 is the major ceramidase responsible for the generation of (dh)SPH and (dh)S1P in the circulation.
Figure 3.
Acer2 knockout reduces SPH, DHS, S1P, and DHS1P levels in whole blood, plasma, serum, erythrocyte, and platelets. A) Whole blood samples were collected into EDTA-coated tubes from Acer2+/+ or Acer2−/− male mice at age 2 mo and were subjected to LC-MS/MS analyses for SPH, DHS, S1P, DHS1P, ceramides (Cer), dihydroceramides (dhCer), sphingomyelins, and monohexasylceramides. Data represent means ± sd (n = 3 mice/genotype). B–D) Platelet-less plasma (B), erythrocytes (C), and platelets (D) were prepared from whole blood samples collected as in panel A and were subjected to LC-MS/MS analysis for SPH, dhSPH, S1P, dhS1P, ceramides, and dihydroceramides. Pi, inorganic phosphate. Data represent means ± sd (n = 3 mice/genotype).
Knocking out Acer2 globally reduces S1P and dhS1P levels in lungs
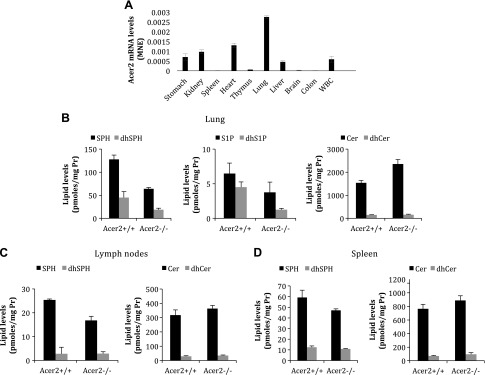
To investigate whether ACER2 plays a role in the regulation of the levels of (dh)S1P in peripheral tissues, we first determined its mRNA levels in major organs in mice. Quantitative PCR analysis found that Acer2 mRNA levels, among the tissues that we examined, are the highest in lungs (Fig. 4A). To assess whether its tissue-specific expression correlates with the role of ACER2 in regulating the tissue levels of (dh)SPH and (dh)S1P, we measured the levels of these sphingolipids in different tissues from Acer2+/+ or Acer2−/− mice. LC-MS/MS revealed that knocking out Acer2 significantly decreased the levels of SPH, dhSPH, S1P, and dhS1P, and increased the levels of ceramides in the lungs (Fig. 4B). Of interest, knocking out Acer2 decreased the levels of SPH and slightly increased the levels of ceramides without affecting the levels of dihydroceramides in lymph nodes (Fig. 4C) or the spleen (Fig. 4D), whereas knocking out Acer2 affected none of these sphingolipids in thymus (Supplemental Fig. 3). Of note, S1P or dhS1P levels were undetectable in these lymphoid tissues from either Acer2+/+ or Acer2−/− mice. These results suggest that ACER2 plays a limited role in the regulation of S1P and dhS1P levels in the lymphoid tissue.
Figure 4.
Acer2 knockout reduces SPH and S1P levels in lungs, but not in the colon, lymph nodes, or thymus. A) Quantitative PCR analysis for Acer2 mRNA levels in major organs in WT mice at age 6 wk. B–D) LC-MS/MS analysis for sphingolipid levels in lungs (B), lymph nodes (C), or spleen (D) in Acer2+/+ or Acer2−/− male mice at age 6 wk. Cer, ceramide; dhCer, dihydroceramide; MNE, mean normalized expression; Pr, protein. Data represent means ± sd (n = 3 mice/genotype).
Knocking out Acer2 specifically from the hematopoietic lineage reduces (dh)SPH and (dh)S1P levels in blood
To assess whether the blood (dh)S1P precursor, (dh)SPH, is generated by the action of Acer2 in hematopoietic cells, bone marrow cells from Acer2−/− or Acer2+/+ mice were reconstituted to lethally irradiated Acer2−/− and Acer2+/+ mice, respectively. At 6 wk post–bone marrow reconstitution, we obtained blood samples and measured (dh)SPH and (dh)S1P levels in the reconstituted animals. Acer2+/+ mice that were reconstituted with bone marrow cells from Acer2+/+ mice demonstrated normal levels of sphingolipids, whereas those reconstituted with bone marrow cells from Acer2−/− mice had low blood levels of SPH (Fig. 5A), dhSPH (Fig. 5A), S1P (Fig. 5B), and dhS1P (Fig. 5B) as Acer2−/− mice reconstituted with bone marrow cells from the Acer2−/− donors. In contrast, delivery of bone marrow cells from Acer2+/+ mice to Acer2−/− mice reconstituted the normal levels of SPH (Fig. 5A), dhSPH (Fig. 5A), S1P (Fig. 5B), and dhS1P (Fig. 5B). Genotyping confirmed that the hematopoietic cells in all recipient mice were successfully reconstituted to those of their corresponding donors (Supplemental Fig. 2B). These results demonstrate that Acer2 expression in hematopoietic cells, but not in peripheral tissues, is predominately responsible for the generation of circulating (dh)SPH and (dh)S1P.
Figure 5.
Hematopoietic cell–specific knockout (KO) of Acer2 markedly reduces SPH, dhSPH, S1P, and dhS1P in whole blood. Acer2+/+ or Acer2−/− mice were lethally irradiated and reconstituted with bone marrow cells from Acer2+/+ or Acer2−/− mice. Whole blood was collected into EDTA-coated tubes and subjected to LC-MS/MS analysis for (dh)SPH (A), (dh)S1P (B), and ceramides (Cer)/dihydroceramides (dhCer; C). WT, Acer2+/+ mice; KO, Acer2−/− mice. Data represent means ± sd (n = 3 mice/genotype).
Knocking out Acer1 or Acer3 does not further reduce blood SPH and S1P in Acer2 knockout mice
As ACER2 may compensate for the loss of function of ACER1 and ACER3 in generating SPH and S1P in Acer1 and Acer3 knockout mice, respectively, we tested whether knocking out Acer1 or Acer3 would further reduce blood SPH and S1P in Acer2−/− mice. We first generated heterozygous Acer1+/−Acer2+/− and Acer2+/−Acer3+/− mice by crossing Acer2−/− mice with Acer1−/− and Acer3−/− mice, respectively. The resultant offspring, Acer1+/−Acer2+/− and Acer2+/−Acer3+/− mice, were then intercrossed to generate homozygous Acer1−/−Acer2−/− and Acer2−/−Acer3−/− offspring. Similar to Acer1−/− mice (33), Acer1−/−Acer2−/− mice started to show a hair loss phenotype at age 2 mo, whereas Acer2−/−Acer3−/− mice showed no overt abnormalities at least during the first 3 mo of age (data not shown). LC-MS/MS demonstrated that Acer1−/−Acer2−/− (Fig. 6A) and Acer2−/−Acer3−/− double-knockout mice (Fig. 6B) had the same blood levels of SPH or S1P as Acer2−/− single-knockout mice, which suggests that Acer2 does not compensate for the loss of function of ACER1 or ACER3 in the generation of SPH as the blood S1P precursor, and confirms that neither ACER1, nor ACER3, plays a role in the generation of SPH as the blood S1P precursor.
Figure 6.
Knocking out Acer1 or Acer3 does not further reduce SPH and S1P levels in Acer2 knockout mice. Whole blood samples were collected into EDTA-coated tubes from Acer1+/+Acer2+/+, Acer1+/+Acer2−/−, Acer1−/−Acer2+/+, Acer1−/−Acer2−/−, Acer2+/+Acer3+/+, Acer2+/+Acer3−/−, Acer2−/−Acer3+/+, and Acer2−/−Acer3−/− mice at age 6 wk and subjected to LC-MS/MS analysis for SPH (A) or S1P levels (B). Data represent means ± sd (n = 3 mice/genotype).
Knocking out Acer2 causes neither hemorrhage, nor lethality in embryos
It has been shown that high blood S1P levels are required for the maturation of embryonic blood vessels, and that its deficiency leads to embryonic hemorrhage and death (16, 42). As knocking out Acer2 alone markedly decreased blood S1P levels, we determined whether blood vessels were defective in Acer2−/− embryos. To this end, we necropsied Acer2+/− dams mated with Acer2+/− males at 14 d postcoitum. Necropsies found that Acer2−/− embryos appeared viable (Fig. 7A) and exhibited no detectable hemorrhage (Fig. 7B), which suggests that Acer2 deficiency in the fetus does not cause defects in embryonic blood vessels.
Figure 7.
Acer2 knockout does not cause hemorrhage in embryos. Pregnant Acer2+/− females mated with Acer2+/− males were euthanized at 15 d postcoitum (dpc), and embryos were inspected for embryo survival (A) and hemorrhage (B). Images represent results of 1 of 4 pregnant females examined.
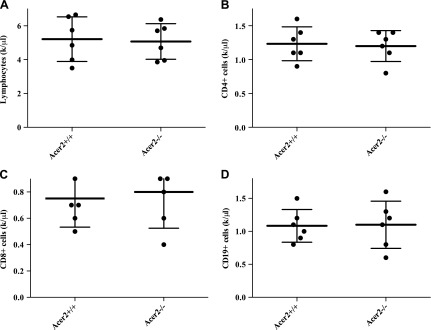
Knocking out Acer2 does not induce lymphopenia in mice
It has been shown that high plasma S1P levels are required for trafficking of lymphocytes from lymphoid organs to peripheral blood; therefore, its deficiency in plasma causes lymphopenia in mice (15). As knocking out Acer2 alone markedly decreased plasma S1P, we next determined whether Acer2−/− mice developed lymphopenia. Of interest, CBC tests revealed that total lymphocytes counts were similar between Acer2−/− and Acer2+/+ mice (Fig. 8A). Consistently, flow cytometry demonstrated that the numbers of peripheral CD4+ T lymphocytes (Fig. 8B), CD8+ T lymphocytes (Fig. 8C), or B19+ B lymphocytes (Fig. 8D) were also similar between Acer2−/− and Acer2+/+ mice. These data suggest that Acer2 knockout seems not to impair lymphocyte egress from lymphoid organs at least in mice at age 3 mo or younger.
Figure 8.
Acer2 knockout does not induce lymphopenia in mice. Blood was withdrawn from Acer2+/+ or Acer2−/− mice at age 5 wk and subjected to CBC tests (A) for total lymphocyte counts or flow cytometric analyses for the average number of peripheral CD3+CD4+ T lymphocytes (B), CD3+CD8+ T lymphocytes (C), or CD19+ B lymphocytes (D). Data represent means ± sd (n = 6 mice/genotype).
DISCUSSION
Previous studies by other groups have indicated that neither the acid ceramidase subtype (30), nor the neutral ceramidase subtype (31) contributes to blood SPH and S1P formation. Our previous study demonstrated that the alkaline ceramidase family is important for the maintenance of high blood levels of S1P in mice (32), but which member in the alkaline ceramidase family plays such a role was unknown. In this study, by using mice that were deficient in each member in the alkaline ceramidase family, we demonstrated that ACER2, but not ACER1 or ACER3, plays a major role in maintaining high blood levels of S1P via the generation of its precursor, SPH, mainly in erythrocytes and platelets. In addition to S1P, we have demonstrated, for the first time to our knowledge, that ACER2 also plays a key role in maintaining high blood levels of dhS1P by controlling the generation of dhSPH in hematopoietic cells in mice. These data collectively suggest that, among the known ceramidases, only Acer2 contributes to the high blood levels of S1P and dhS1P in mice.
The S1P precursor, SPH, cannot be synthesized via an anabolic pathway in mammalian cells, and thus must be generated via a catabolic pathway, the hydrolysis of ceramides. This is via the action of the acid, neutral, and alkaline ceramidase subtypes that are encoded by 5 distinct genes: ASAH1, ASAH2, ACER1, ACER2, and ACER3 in humans, and Asah1, Asah2, Acer1, Acer2, and Acer3 in mice (28, 29). Our in vitro studies strongly suggest that the generation of SPH by the action of ceramidases is the rate-limiting step for S1P formation (32), although SPHK1 and SPHK2 are essential for S1P formation in tissues (16). In this study, we have demonstrated that knocking out Acer2 markedly reduces the blood levels of both SPH and S1P in mice. In contrast, knocking out Acer1 or Acer3 does not alter the blood levels of SPH or S1P in mice. Here, we have shown that this is not a result of a compensatory effect by ACER2, as knocking out neither Acer1, nor Acer3, further reduces blood SPH or S1P levels in Acer2-deficient mice. These studies suggest that, in the alkaline ceramidase family, ACER2 is the only member that plays a role in maintaining high blood levels of S1P in mice via the generation of its precursor, SPH. Previous studies have shown that knocking out Asah1 (30) or Asah2 (31) does not alter blood SPH and S1P levels in mice, and it remains unclear whether the lack of change in the blood levels of SPH and S1P in Asah1- or Asah2-deficient mice is a result of a compensatory effect by ACER2. If not, the remainder of the blood S1P precursor, SPH, in Acer2−/− mice either comes from diet or is generated via an unidentified metabolic pathway. The former scenario is unlikely as we found that fasting did not further reduce the blood levels of SPH (Supplemental Fig. 3G) and S1P (Supplemental Fig. 3H) in Acer2−/− mice. In addition to a marked decrease in SPH and S1P levels in the blood, Acer2 knockout also caused a significant decrease in the levels of ceramides in plasma and platelets. This result is contradictory to the role of ACER2 as an enzyme that is responsible for the hydrolysis of ceramides. This unexpected result could be caused by a compensatory up-regulation of an enzyme that is responsible for the conversion of ceramides to other sphingolipids or a feedback inhibition of an enzyme responsible for the formation of ceramides. The latter scenario may be the case as we demonstrated that knocking out Acer2 also decreased blood levels of dhSPH and dihydroceramides—precursors of ceramides—without affecting sphingomyelins (Supplemental Fig. 3C) and monohexosylceramides (Supplemental Fig. 3D). Acer2 knockout reduces the levels of dhSPH, dihydroceramides, and ceramides, likely by inhibiting serine palmitoyl-CoA transferase, which catalyzes the initial step of sphingolipid biosynthesis. In addition to C18SPH, the major sphingoid base in mouse blood, the minor sphingoid base C20SPH, but not C16SPH or C17SPH, was detected, and Acer2 knockout significantly reduced the blood levels of C20SPH (Supplemental Fig. 3I). Taken together, data from this and previous studies strongly suggest that, among the known ceramidases, Acer2 is a major ceramidase that is responsible for the maintenance of high blood S1P and dhS1P levels in mice.
Previous studies have suggested that erythrocytes are the major source of blood S1P (8), but the source of SPH that serves as the blood S1P precursor remained unclear. In this study, we demonstrated that Acer2 knockout specifically from hematopoietic cells via bone marrow transplantation reduced the blood levels of both SPH and S1P to a degree that was similar to that observed with Acer2 whole-body knockout animals, which suggests that the blood S1P precursor, SPH, is generated mainly in hematopoietic cells, especially by the action of Acer2. This conclusion is firmly supported by the finding that knocking out Acer2 also markedly decreased the levels of both SPH and S1P in erythrocytes and platelets.
Previous studies have indicated that S1P levels are low or undetectable in most peripheral tissues. Consistent with this notion, we demonstrated that S1P levels were much lower in the lung than in the blood, and were undetectable in the lymphoid tissues thymi, lymph nodes, and spleen. S1P levels were detectable in the lungs, but not in lymphoid tissues, likely because Acer2 mRNA levels are significantly higher in the lungs than in lymphoid tissue. Indeed, we demonstrated that knocking out ACER2 markedly reduces both S1P and SPH levels in the lungs. Low ACER2 expression in lymphoid tissue should facilitate the formation of S1P concentration gradients between the lymphoid tissue and plasma.
S1P has been shown to play a key role in many important biologic processes by binding and activating 1 or more of 5 specific GPCRs, S1P1–5. Knocking out both Sphk1 and Sphk2 globally (16) or specifically from erythrocytes (42) abolishes plasma S1P, which results in embryonic lethality as a result of hemorrhage, suggesting that plasma S1P is essential for vascular development and fetal survival. Knocking out Acer2 here does not cause embryonic lethality as the intercross of Acer2+/− mice produces the same number of live-born neonates as the intercross of Acer2+/+ mice. Consistently, Acer2−/− embryos were viable and exhibited no detectable hemorrhage. These results indicate that Acer2 deficiency alone does not affect vascular development and maturation, and that a small fraction (∼35% of WT levels) of plasma S1P is sufficient for vascular development and fetal survival. This notion is supported by the finding that knocking out Sphk1 alone neither impairs vascular development, nor results in embryonic lethality, even though serum S1P is reduced by 50% in Sphk1 knockout mice (10); however, it remains unclear whether the loss of Acer2 impairs the fitness of the cardiovascular system in adult mice under pathologic conditions.
S1P concentration gradients between blood and lymphoid tissues are essential for trafficking T and B lymphocytes from lymphoid tissues to the circulation (15, 43). Indeed, either increasing the levels of S1P in lymphoid tissues by inhibiting or knocking out the S1P lyase SGPL activity (43, 44), or depleting plasma S1P by knocking out both Sphk1 and Sphk2 (15) induces lymphopenia in mice. In this study, we found that the numbers of peripheral T or B lymphocytes were similar between Acer2−/− and Acer2+/+ mice, which suggests that the loss of Acer2 seems not to affect lymphocyte trafficking, at least in young mice. In support of this idea, Allende et al. (10) reported that Sphk1 deficiency does not cause lymphopenia in young mice, although plasma levels are reduced by 50%. These results suggest that a 70% reduction in plasma S1P does not impair the S1P concentration gradient between blood and lymphoid organs. Indeed, we demonstrated that S1P levels are still much higher in plasma than in the lymphoid tissue in Acer2−/− mice, although their plasma S1P levels are significantly reduced compared with Acer2+/+ mice.
Although S1P is abundant in the circulation, no studies have demonstrated its role in hematopoiesis. Our CBC tests found that, like lymphocytes, the loss of Acer2 does not affect the number of other blood cell types, including erythrocytes and platelets (Supplemental Fig. 4), although S1P levels are markedly reduced in these anucleated cells, which suggests that ACER2 may not play a role in hematopoiesis.
Previous studies have demonstrated conflicting results with regard to the role of S1P in platelet aggregation. Gazit et al. (17) demonstrated that reducing plasma S1P levels by knocking out both Sphk1 and Sphk2 inhibited mouse platelet aggregation, whereas Onuma et al. (45) found that S1P, when added exogenously, also inhibited collagen-induced human platelet aggregation (45). As platelet aggregation is important in hemostasis (46), we assessed whether Acer2 knockout affects the duration of bleeding as a result of a tail injury in mice. We demonstrated that bleeding time was similar between Acer2+/+ and Acer2−/− mice (Supplemental Fig. 5), which suggests that the loss of Acer2 does not affect the function of platelets in hemostasis.
In summary, we have demonstrated that the mouse alkaline ceramidase, ACER2, plays a key role in maintaining high blood levels of S1P and dhS1P in mice by controlling the generation of SPH and dhSPH, respectively, in hematopoietic cells, erythrocytes, and platelets in particular. S1P has been shown to play causal roles in the pathogenesis of many inflammatory and cardiovascular diseases (3). We suggest that targeting ACER2 would be a safe approach to lower blood levels of S1P, as Acer2 deficiency does not seem to result in major pathologies in mice, at least at young ages. Therefore, we envision that inhibiting the ACER2/SPH/S1P pathway may represent a novel therapeutic approach for the treatment of S1P-associated inflammatory and cardiovascular diseases.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Peter M. Kutny (Genetic Engineering Technologies, The Jackson Laboratory) for assistance with microinjections, Cindy Avery at the Jackson Laboratory for animal maintenance and genotyping, and Ping Ji (State University of New York at Stony Brook) for assistance with histological analysis. The authors also thank Dr. Ye Chen (Southern Medical University) for assistance with animal studies. This work was supported, in whole or in part, by U.S. National Institutes of Health, National Cancer Institute Grants R01-CA163825 (to C.M.) and P01-CA097132 (to Y.A.H. and C.M.). This work was also supported by the Sphingolipid Animal Cancer Pathobiology Shared Resource Core. The authors declare no conflicts of interest.
Glossary
- ACER
alkaline ceramidase
- ASAH
acid ceramidase
- CBC
complete blood count
- dhS1P
dihydrosphingosine-1-phophate
- dhSPH
dihydrosphingosine
- IS
internal standard
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- S1P
sphingosine-1-phosphate
- SBP
sphingoid base-1- phosphate
- sgRNA
single-guide RNA
- SPH
sphingosine
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
F. Li, R. Xu, M. V. Wiles, and C. Mao designed research; F. Li, R. Xu, B. E. Low, C.-L. Lin, M. Garcia-Barros, J. Schrandt, I. Mileva, A. Snider, X.-C. Jiang, and C. K. Luo performed research; F. Li, R. Xu, and C. Mao analyzed data; M.-S. Li, Y. A. Hannun, and L. M. Obeid contributed new reagents; and F. Li, R. Xu, and C. Mao wrote the paper.
REFERENCES
- 1.Mendelson K., Evans T., Hla T. (2014) Sphingosine 1-phosphate signalling. Development 141, 5–9 10.1242/dev.094805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chun J., Hla T., Lynch K. R., Spiegel S., Moolenaar W. H. (2010) International union of basic and clinical pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 62, 579–587 10.1124/pr.110.003111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Proia R. L., Hla T. (2015) Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J. Clin. Invest. 125, 1379–1387 10.1172/JCI76369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pyne N. J., Pyne S. (2017) Sphingosine 1-phosphate receptor 1 signaling in mammalian cells. Molecules 22, E344 10.3390/molecules22030344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strub G. M., Maceyka M., Hait N. C., Milstien S., Spiegel S. (2010) Extracellular and intracellular actions of sphingosine-1-phosphate. Adv. Exp. Med. Biol. 688, 141–155 10.1007/978-1-4419-6741-1_10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvarez S. E., Harikumar K. B., Hait N. C., Allegood J., Strub G. M., Kim E. Y., Maceyka M., Jiang H., Luo C., Kordula T., Milstien S., Spiegel S. (2010) Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 465, 1084–1088 10.1038/nature09128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hait N. C., Allegood J., Maceyka M., Strub G. M., Harikumar K. B., Singh S. K., Luo C., Marmorstein R., Kordula T., Milstien S., Spiegel S. (2009) Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325, 1254–1257 10.1126/science.1176709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hänel P., Andréani P., Gräler M. H. (2007) Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 21, 1202–1209 10.1096/fj.06-7433com [DOI] [PubMed] [Google Scholar]
- 9.Moritz E., Wegner D., Gross S., Bahls M., Dorr M., Felix S. B., Ittermann T., Oswald S., Nauck M., Friedrich N., Boger R. H., Daum G., Schwedhelm E., Rauch B. H. (2017) Reference intervals for serum sphingosine-1-phosphate in the population-based study of health in pomerania. Clin. Chim. Acta. 468, 25–31 [DOI] [PubMed] [Google Scholar]
- 10.Allende M. L., Sasaki T., Kawai H., Olivera A., Mi Y., van Echten-Deckert G., Hajdu R., Rosenbach M., Keohane C. A., Mandala S., Spiegel S., Proia R. L. (2004) Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem. 279, 52487–52492 10.1074/jbc.M406512200 [DOI] [PubMed] [Google Scholar]
- 11.Cyster J. G., Schwab S. R. (2012) Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 30, 69–94 10.1146/annurev-immunol-020711-075011 [DOI] [PubMed] [Google Scholar]
- 12.Cyster J. G. (2005) Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23, 127–159 10.1146/annurev.immunol.23.021704.115628 [DOI] [PubMed] [Google Scholar]
- 13.Rosen H., Goetzl E. J. (2005) Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat. Rev. Immunol. 5, 560–570 10.1038/nri1650 [DOI] [PubMed] [Google Scholar]
- 14.Rosen H., Sanna G., Alfonso C. (2003) Egress: a receptor-regulated step in lymphocyte trafficking. Immunol. Rev. 195, 160–177 10.1034/j.1600-065X.2003.00068.x [DOI] [PubMed] [Google Scholar]
- 15.Pappu R., Schwab S. R., Cornelissen I., Pereira J. P., Regard J. B., Xu Y., Camerer E., Zheng Y. W., Huang Y., Cyster J. G., Coughlin S. R. (2007) Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 316, 295–298 10.1126/science.1139221 [DOI] [PubMed] [Google Scholar]
- 16.Mizugishi K., Yamashita T., Olivera A., Miller G. F., Spiegel S., Proia R. L. (2005) Essential role for sphingosine kinases in neural and vascular development. Mol. Cell. Biol. 25, 11113–11121 10.1128/MCB.25.24.11113-11121.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gazit S. L., Mariko B., Thérond P., Decouture B., Xiong Y., Couty L., Bonnin P., Baudrie V., Le Gall S. M., Dizier B., Zoghdani N., Ransinan J., Hamilton J. R., Gaussem P., Tharaux P. L., Chun J., Coughlin S. R., Bachelot-Loza C., Hla T., Ho-Tin-Noé B., Camerer E. (2016) Platelet and erythrocyte sources of S1P are redundant for vascular development and homeostasis, but both rendered essential after plasma S1P depletion in anaphylactic shock. Circ. Res. 119, e110–e126 10.1161/CIRCRESAHA.116.308929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y., Wada R., Yamashita T., Mi Y., Deng C. X., Hobson J. P., Rosenfeldt H. M., Nava V. E., Chae S. S., Lee M. J., Liu C. H., Hla T., Spiegel S., Proia R. L. (2000) Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 106, 951–961 10.1172/JCI10905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cutignano A., Chiuminatto U., Petruzziello F., Vella F. M., Fontana A. (2010) UPLC-MS/MS method for analysis of sphingosine 1-phosphate in biological samples. Prostaglandins Other Lipid Mediat. 93, 25–29 10.1016/j.prostaglandins.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 20.Véret J., Coant N., Gorshkova I. A., Giussani P., Fradet M., Riccitelli E., Skobeleva A., Goya J., Kassis N., Natarajan V., Portha B., Berdyshev E. V., Le Stunff H. (2013) Role of palmitate-induced sphingoid base-1-phosphate biosynthesis in INS-1 β-cell survival. Biochim. Biophys. Acta 1831, 251–262 10.1016/j.bbalip.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 21.Barth B. M., Shanmugavelandy S. S., Kaiser J. M., McGovern C., Altınoğlu E. I., Haakenson J. K., Hengst J. A., Gilius E. L., Knupp S. A., Fox T. E., Smith J. P., Ritty T. M., Adair J. H., Kester M. (2013) PhotoImmunoNanoTherapy reveals an anticancer role for sphingosine kinase 2 and dihydrosphingosine-1-phosphate. ACS Nano 7, 2132–2144 10.1021/nn304862b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Callihan P., Zitomer N. C., Stoeling M. V., Kennedy P. C., Lynch K. R., Riley R. T., Hooks S. B. (2012) Distinct generation, pharmacology, and distribution of sphingosine 1-phosphate and dihydrosphingosine 1-phosphate in human neural progenitor cells. Neuropharmacology 62, 988–996 10.1016/j.neuropharm.2011.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bu S., Kapanadze B., Hsu T., Trojanowska M. (2008) Opposite effects of dihydrosphingosine 1-phosphate and sphingosine 1-phosphate on transforming growth factor-beta/Smad signaling are mediated through the PTEN/PPM1A-dependent pathway. J. Biol. Chem. 283, 19593–19602 10.1074/jbc.M802417200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohama T., Olivera A., Edsall L., Nagiec M. M., Dickson R., Spiegel S. (1998) Molecular cloning and functional characterization of murine sphingosine kinase. J. Biol. Chem. 273, 23722–23728 10.1074/jbc.273.37.23722 [DOI] [PubMed] [Google Scholar]
- 25.Liu H., Sugiura M., Nava V. E., Edsall L. C., Kono K., Poulton S., Milstien S., Kohama T., Spiegel S. (2000) Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 275, 19513–19520 10.1074/jbc.M002759200 [DOI] [PubMed] [Google Scholar]
- 26.Zhou J., Saba J. D. (1998) Identification of the first mammalian sphingosine phosphate lyase gene and its functional expression in yeast. Biochem. Biophys. Res. Commun. 242, 502–507 10.1006/bbrc.1997.7993 [DOI] [PubMed] [Google Scholar]
- 27.Venkataraman K., Lee Y. M., Michaud J., Thangada S., Ai Y., Bonkovsky H. L., Parikh N. S., Habrukowich C., Hla T. (2008) Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 102, 669–676 10.1161/CIRCRESAHA.107.165845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao C., Obeid L. M. (2008) Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim. Biophys. Acta 1781, 424–434 10.1016/j.bbalip.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coant N., Sakamoto W., Mao C., Hannun Y. A. (2017) Ceramidases, roles in sphingolipid metabolism and in health and disease. Adv. Biol. Regul. 63, 122–131 10.1016/j.jbior.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dworski S., Lu P., Khan A., Maranda B., Mitchell J. J., Parini R., Di Rocco M., Hugle B., Yoshimitsu M., Magnusson B., Makay B., Arslan N., Guelbert N., Ehlert K., Jarisch A., Gardner-Medwin J., Dagher R., Terreri M. T., Lorenco C. M., Barillas-Arias L., Tanpaiboon P., Solyom A., Norris J. S., He X., Schuchman E. H., Levade T., Medin J. A. (2017) Acid ceramidase deficiency is characterized by a unique plasma cytokine and ceramide profile that is altered by therapy. Biochim. Biophys. Acta 1863, 386–394 10.1016/j.bbadis.2016.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snider A. J., Wu B. X., Jenkins R. W., Sticca J. A., Kawamori T., Hannun Y. A., Obeid L. M. (2012) Loss of neutral ceramidase increases inflammation in a mouse model of inflammatory bowel disease. Prostaglandins Other Lipid Mediat. 99, 124–130 10.1016/j.prostaglandins.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu R., Sun W., Jin J., Obeid L. M., Mao C. (2010) Role of alkaline ceramidases in the generation of sphingosine and its phosphate in erythrocytes. FASEB J. 24, 2507–2515 10.1096/fj.09-153635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin C. L., Xu R., Yi J. K., Li F., Chen J., Jones E. C., Slutsky J. B., Huang L., Rigas B., Cao J., Zhong X., Snider A. J., Obeid L. M., Hannun Y. A., Mao C. (2017) Alkaline ceramidase 1 protects mice from premature hair loss by maintaining the homeostasis of hair follicle stem cells. Stem Cell Reports 9, 1488–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang K., Xu R., Schrandt J., Shah P., Gong Y. Z., Preston C., Wang L., Yi J. K., Lin C. L., Sun W., Spyropoulos D. D., Rhee S., Li M., Zhou J., Ge S., Zhang G., Snider A. J., Hannun Y. A., Obeid L. M., Mao C. (2015) Alkaline ceramidase 3 deficiency results in purkinje cell degeneration and cerebellar ataxia due to dyshomeostasis of sphingolipids in the brain. PLoS Genet. 11, e1005591 10.1371/journal.pgen.1005591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu Y., Sander J. D., Reyon D., Cascio V. M., Joung J. K. (2014) Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat. Biotechnol. 32, 279–284 10.1038/nbt.2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Low B. E., Kutny P. M., Wiles M. V. (2016) Simple, efficient CRISPR-Cas9-mediated gene editing in mice: strategies and methods. Methods Mol. Biol. 1438, 19–53 10.1007/978-1-4939-3661-8_2 [DOI] [PubMed] [Google Scholar]
- 37.Truett G. E., Heeger P., Mynatt R. L., Truett A. A., Walker J. A., Warman M. L. (2000) Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 29, 52, 54. [DOI] [PubMed] [Google Scholar]
- 38.Cui Y. Z., Hisha H., Yang G. X., Fan T. X., Jin T., Li Q., Lian Z., Ikehara S. (2002) Optimal protocol for total body irradiation for allogeneic bone marrow transplantation in mice. Bone Marrow Transplant. 30, 843–849 10.1038/sj.bmt.1703766 [DOI] [PubMed] [Google Scholar]
- 39.Muller P. Y., Janovjak H., Miserez A. R., Dobbie Z. (2002) Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques 32, 1372–1374, 1376, 1378–1379 [PubMed] [Google Scholar]
- 40.Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J. A., Charpentier E. (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 10.1126/science.1225829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cong L., Ran F. A., Cox D., Lin S., Barretto R., Habib N., Hsu P. D., Wu X., Jiang W., Marraffini L. A., Zhang F. (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 10.1126/science.1231143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong Y., Yang P., Proia R. L., Hla T. (2014) Erythrocyte-derived sphingosine 1-phosphate is essential for vascular development. J. Clin. Invest. 124, 4823–4828 10.1172/JCI77685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwab S. R., Pereira J. P., Matloubian M., Xu Y., Huang Y., Cyster J. G. (2005) Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309, 1735–1739 10.1126/science.1113640 [DOI] [PubMed] [Google Scholar]
- 44.Vogel P., Donoviel M. S., Read R., Hansen G. M., Hazlewood J., Anderson S. J., Sun W., Swaffield J., Oravecz T. (2009) Incomplete inhibition of sphingosine 1-phosphate lyase modulates immune system function yet prevents early lethality and non-lymphoid lesions. PLoS One 4, e4112 10.1371/journal.pone.0004112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Onuma T., Tanabe K., Kito Y., Tsujimoto M., Uematsu K., Enomoto Y., Matsushima-Nishiwaki R., Doi T., Nagase K., Akamatsu S., Tokuda H., Ogura S., Iwama T., Kozawa O., Iida H. (2017) Sphingosine 1-phosphate (S1P) suppresses the collagen-induced activation of human platelets via S1P4 receptor. Thromb. Res. 156, 91–100 10.1016/j.thromres.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Andrews M., Yang Y., Lang S., Jin J. W., Cameron-Vendrig A., Zhu G., Reheman A., Ni H. (2012) Platelets in thrombosis and hemostasis: old topic with new mechanisms. Cardiovasc. Hematol. Disord. Drug Targets 12, 126–132 10.2174/1871529X11202020126 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.