Fig. 3.
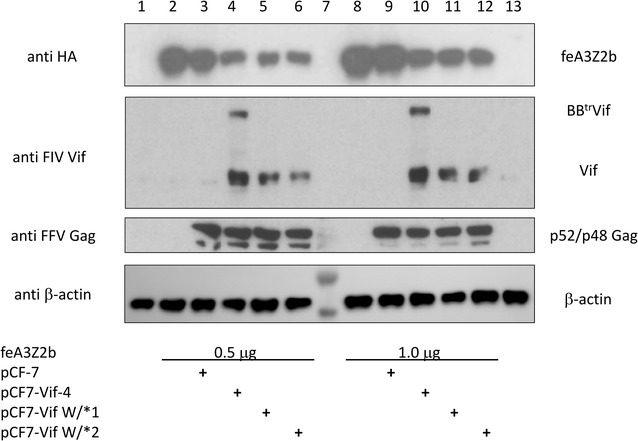
Reduced steady state levels of feA3Z2b in FIV Vif- and BettrVif-expressing cells. Parental wild-type FFV full-length pCF-7 genome and FFV-Vif chimeric clones pCF-Vif-4, pCF7-Vif W/*1, and -W/*2 were transfected into HEK 293T cells together with 0.5 (Fig. 3, lanes 2 to 6) or 1.0 μg (lanes 8 to 12) of a plasmid encoding HA-tagged feA3Z2b as indicated below the blots. Cells transfected with the plasmid encoding feA3Z2b and pcDNA, as well as pcDNA-transfected cells served as controls (lanes 2 and 8, and 1 and 13, respectively). Cells were lysed 2 d after transfection and 20 μg total of each protein lysate was subjected to immunoblotting against HA (detecting HA-tagged feA3Z2b), FIV Vif, FFV Gag and β-actin (from top to bottom and indicated at the left). Lane 7 was loaded with a pre-stained protein marker. The bands corresponding to apparent molecular masses of 40 and about 55 kDa are seen below and above the β-actin of 42 kDa (bottom panel developed in an Intas ECL Chemocam Imaging device). All other blots were exposed to autoradiography films and thus, pre-stained protein markers are not visible in lane 7. The names of proteins specifically detected by immunoblotting are given at the right-hand side
