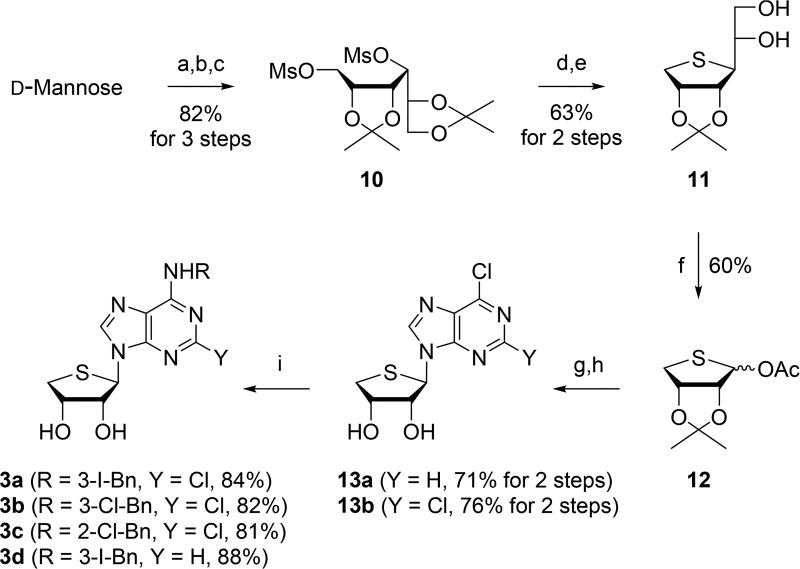
Scheme 2.
Synthesis of Truncated 4′-Thioadenosine Derivatives 3a–d29,a
aReagents and conditions: (a) 2,2-dimethoxypropane, camphosulfonic acid, acetone, rt, 15 h, 95%; (b) NaBH4, EtOH, rt, 2 h, 92%; (c) MsCl, Et3N, CH2Cl2, rt, 1 h, 94%; (d) Na2S, DMF, 80 °C, 15 h, 78%; (e) 60% AcOH, rt, 2 h, 81%; (f) Pb(OAc)4, EtOAc, rt, 15 h, 60%; (g) 6-chloropurine for 13a, 2,6-dichloropurine for 13b, ammonium sulfate, HMDS, 170 °C, 15 h, then TMSOTf, DCE, rt to 80 °C, 3 h; (h) 2 N HCl, THF, rt, 15 h; (i) RNH2, Et3 N, EtOH, rt, 1–3 d.