Figure 5. The ITK PHTH/Kinase interaction is selectively disrupted by binding of soluble inositol 1,3,4,5-tetrakisphosphate (IP(1,3,4,5)P4).
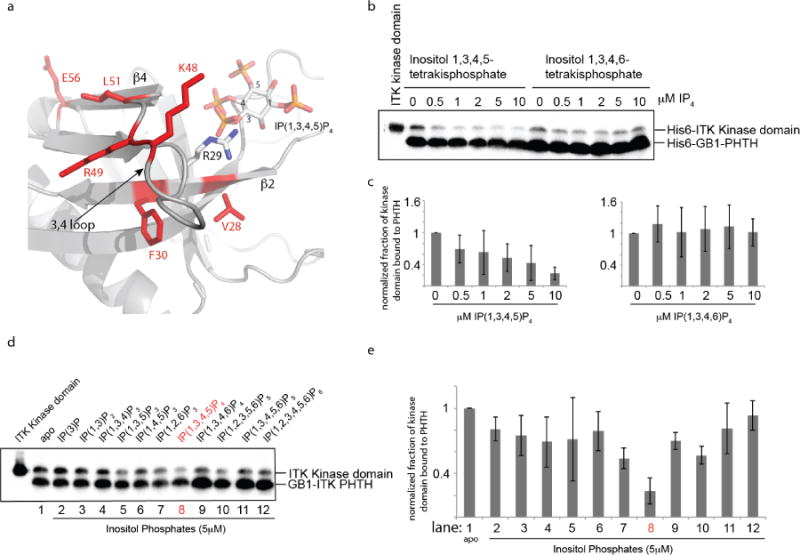
(a) Close up view of the ITK PHTH interface residues that mediate contact to the ITK kinase domain (labeled and in red) and the adjacent lipid binding site. The computational model of the ITK PHTH domain was aligned with the crystal structure of BTK PHTH domain bound to IP(1,3,4,5)P4 (PDB ID: 1B55). BTK PHTH domain is not shown for clarity. The IP4 head group of PIP3 is shown bound to the PHTH domain and positions 1,3,4, and 5 are labeled. The position of the conserved arginine that binds IP4 is indicated to show that it sits between two residues that mediate contacts to the ITK kinase domain (V28 and F30). (b) Anti-His6 blots showing ITK kinase domain (4μM) binding to the ITK PHTH domain (2μM) immobilized on IgG sepharose beads in the presence of soluble inositol 1,3,4,5-tetrakisphosphate (IP(1,3,4,5)P4) and the regioisomeric IP4, inositol 1,3,4,6-tetrakisphosphate (IP(1,3,4,6)P4). The first lane shows total ITK kinase domain input followed by increasing concentration of IP4 compounds (0-10 μM). (c) Histogram representation of the fraction of ITK kinase domain bound to the ITK PHTH domain at 0, 0.5, 1, 2, 5 and 10 μM IP4 (data for inositol 1,3,4,5-tetrakisphosphate is on the left and inositol 1,3,4,6-tetrakisphosphate is on the right). Bound kinase is normalized to His6-GB1 PHTH level in each lane and then compared to the amount of bound kinase in the absence of IP4 (0 μM). (d,e) The same experiment described for panels (b) and (c) is carried out for a panel of inositol phosphates. Lane 1 (apo) contains no added inositol phosphate and the specific compound used in lanes 2-12 is indicated above the blot in (d). The concentration of all inositol phosphates is 5 μM. The IP4 compound that is the soluble head group of the PIP3 target of ITK in T cells (inositol 1,3,4,5-tetrakisphosphate) is labeled in red.
