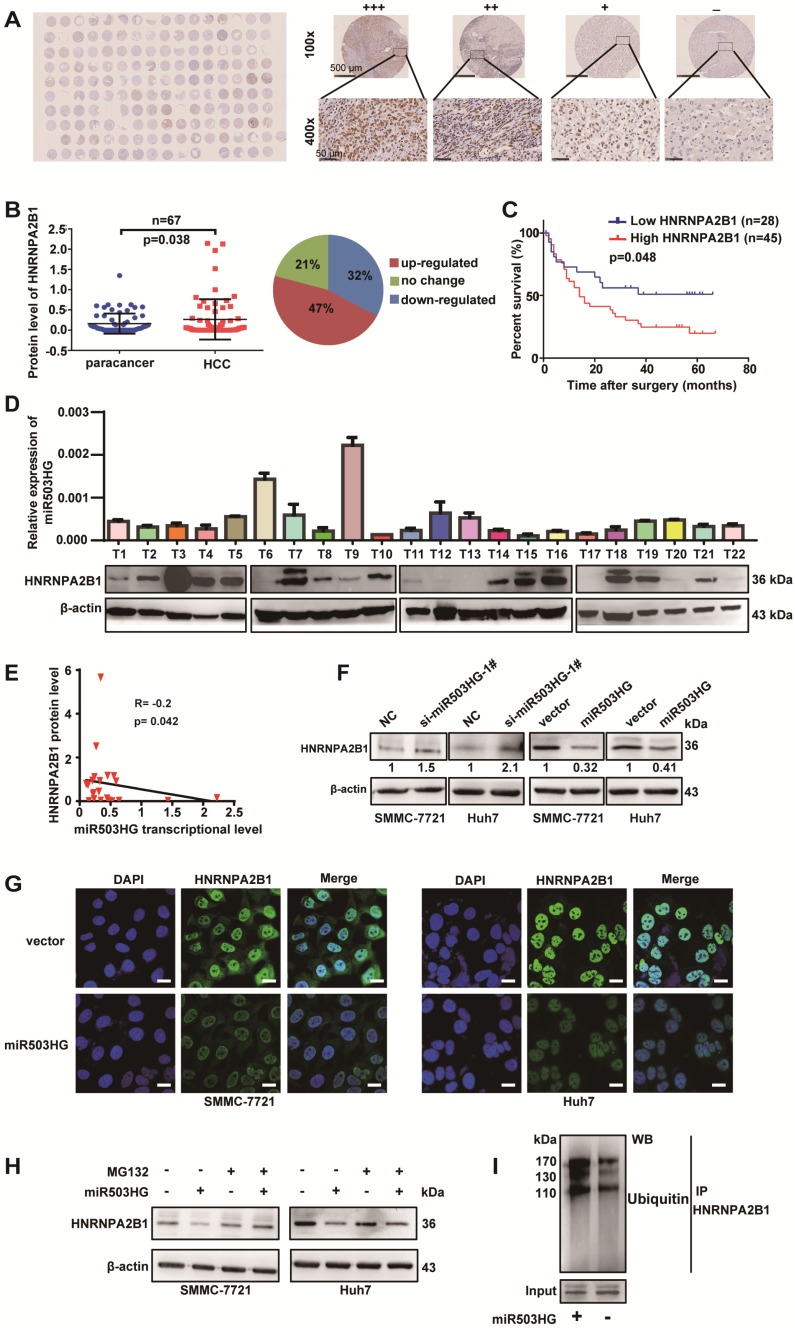
Figure 4.
miR503HG mediates ubiquitination and degradation of HNRNPA2B1 in HCC cells. (A) A tissue microarray with 80 pairs of HCC and corresponding noncancerous liver tissues was used to determine the protein expression level of HNRNPA2B1. (B) The protein level of HNRNPA2B1 was significantly increased in 47% of HCC tissues. (C) Kaplan-Meier analysis for OS was performed according to HNRNPA2B1 levels. (D) The transcriptional level of miR503HG and the protein expression level of HNRNPA2B1 in 22 HCC tissues. (E) Correlation analysis showed a negative relationship between miR503HG (x) and HNRNPA2B1 (y) in 22 HCC tissues (R = -0.2, P = 0.042). miR503HG and HNRNPA2B1 expression was normalized to β-actin. (F) HNRNPA2B1 protein expression in HCC cells with miR503HG knockdown or overexpression. (G) Immunofluorescence was used to compare the expression levels of HNRNPA2B1 between SMMC-7721 (or Huh7) cells with miR503HG overexpression and control cells. Scale bar = 20 μm. (H) SMMC-7721 and Huh7 cells expressing either miR503HG or control vector were cultured in the presence or absence of MG132 (20 μM) for 6 h. Cell lysates were then analyzed by western blotting. (I) Lysates from SMMC-7721 cells with miR503HG overexpression that were treated with MG132 were subjected to immunoprecipitation with anti-HNRNPA2B1 antibody followed by immunoblotting analysis with anti-ubiquitin or anti-HNRNPA2B1 antibody.