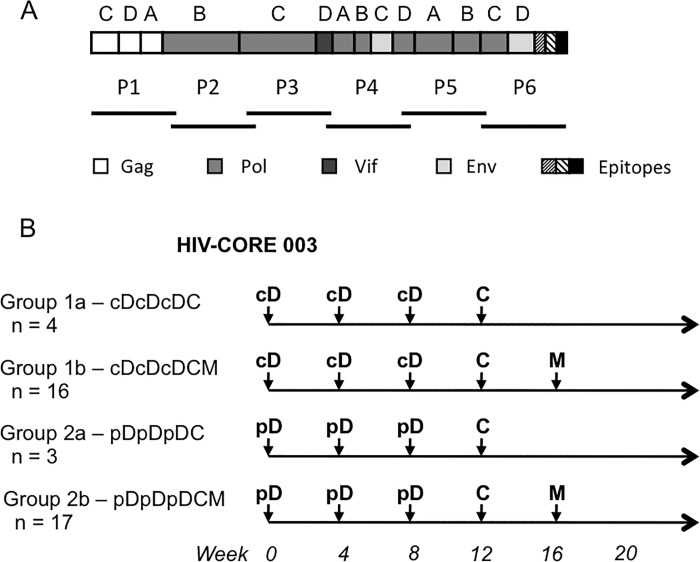
Fig 2. Vaccine immunogen HIVconsv and the design of the HIV-CORE 003 trial.
(A) Schematic representations of the HIVconsv immunogen and six pools P1-P6 of a total of 199 overlapping peptides used for the detection of the human vaccine-elicited T-cell responses. HIVconsv is a chimaeric protein assembled from 14 highly conserved regions of the HIV-1 proteome, the HIV-1 protein origins of which are colour-coded below. (B) Volunteers in the phase I/IIa HIV-CORE 003 trial were randomized into Group 1 for depletion of serum amyloid P component (SAP) by a 26-hour infusion of drug CPHPC, or Group 2 receiving saline infusion alone as placebo, whereby the DNA was injected after 24 hours of infusion. All volunteers were boosted with non-replicating recombinant virus vaccines expressing the same HIVconsv immunogen as indicated. cD–pSG2.HIVconsv DNA with CPHPC infusion prior to DNA administration; pD–pSG2.HIVconsv with placebo infusion prior to DNA; C–non-replicating simian (chimpanzee) adenovirus-vectored vaccine ChAdV63.HIVconsv; and M–non-replicating poxvirus-vectored vaccine MVA.HIVconsv. The number of volunteers recruited into each study group is indicated.