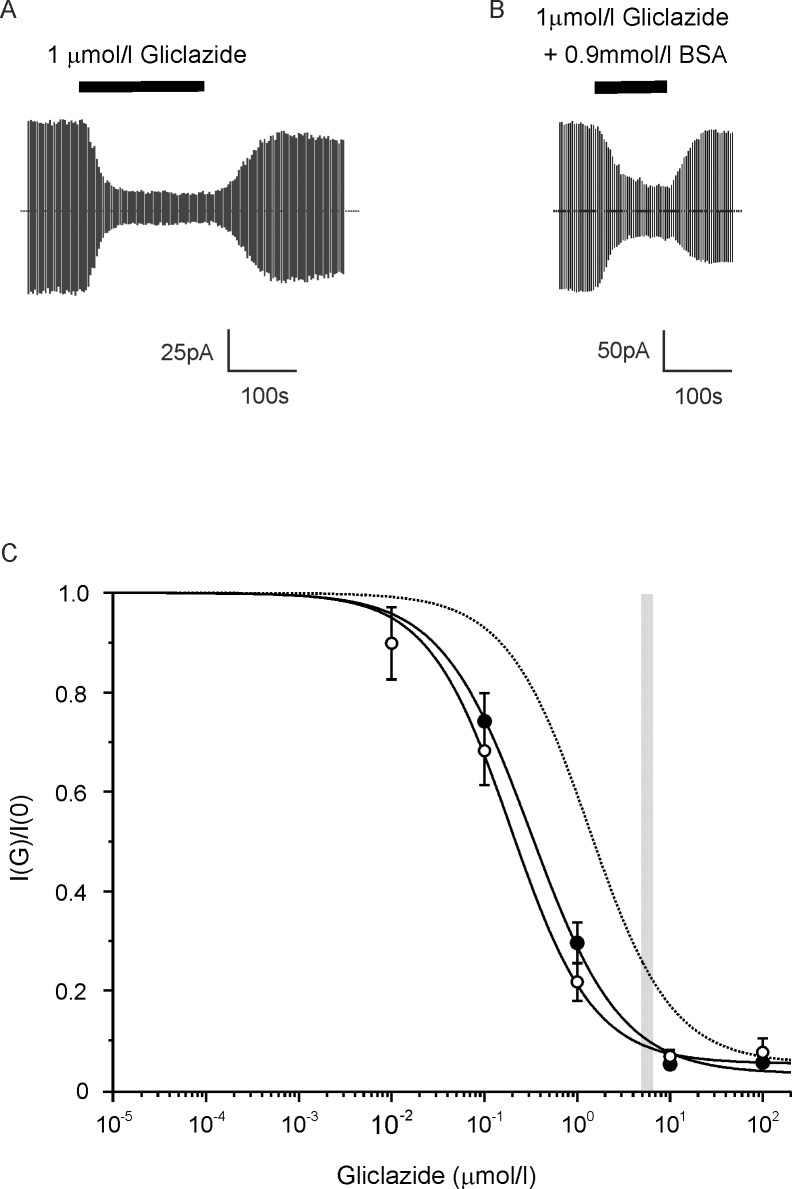
Fig 1. Effect of BSA on gliclazide block of the β-cell KATP current.
(A,B) Representative whole-cell KATP currents recorded from mouse pancreatic β-cells in response to alternating ±20mV steps from a holding potential of -70mV. Gliclazide (1μmol/l) was added (as indicated by the bars) in the absence (A) or presence (B) of 0.9mmol/l BSA. The dotted line indicates the zero current level. (C) Concentration-response relationships for gliclazide inhibition of whole-cell KATP currents in mouse β-cells in the absence (○, n = 6) or presence (●, n = 5) of 0.9mmol/l BSA. Current is expressed relative to that in the absence of gliclazide. The solid lines are the best fit of Eq 2 to the mean data: (○) IC50 = 190nmol/l, h = 0.97, a = 0.05; (●) IC50 = 320nmol/l, h = 0.90, a = 0.03. This shift predicts 60% of drug is bound and a dissociation constant (Kd) for drug binding to BSA of ~1.3mmol/l (Eq 3). The dotted line is the estimated gliclazide block in the presence of human plasma (HP) assuming gliclazide binds to plasma proteins with a Kd of 155μmol/l. The width of the grey bar indicates the mean CSS±SEM (steady-state total plasma concentrations of sulphonylurea drugs) of the total gliclazide concentration in the plasma estimated from a daily dose of 80mg and an AUC (the area under the plasma concentration against time curve) of 44μg.h/ml [26].