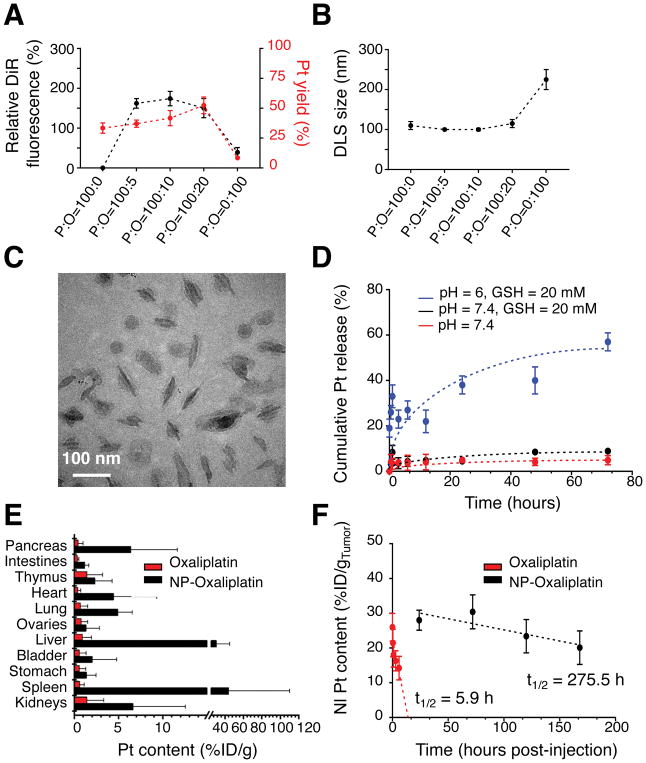
Figure 5. Characterization and delivery of oxaliplatin-loaded PEO-b-PCL-based worm-like micelles.
(A) Relative DiR fluorescence intensities and the loading efficiencies of a lipophilic oxaliplatin(IV) prodrug (based on platinum (Pt) content) in PEO-b-PCL-based worm-like micelles as a function of the initial weight ratio of polymer (P) to oxaliplatin (O) used for nanoparticle incorporation (P:O ratio). (B) DLS of worm-like micelles as a function of the initial P:O ratio. (C) Cryo-TEM micrograph of worm-like micelles that are maximally loaded with the lipophilic oxaliplatin(IV) prodrug (and that were formed at an initial P:O = 100:10). (D) In situ release of free oxaliplatin (based on Pt) from oxaliplatin(IV)-loaded PEO-b-PCL-based worm-like micelles under various environmental conditions. (E) Pt content in excised organs based on the injected dose of oxaliplatin per gram tissue (%ID/g) and as assessed at 24 h after intravenous (tail-vein) injection of either free oxaliplatin or oxaliplatin(IV)-loaded PEO-b-PCL-based worm-like micelles in the autochthonous KPC model. Note that the pancreas is the major organ wherein the tumors are located in these mice; hence, it serves as a surrogate to evaluate the extent of intratumoral accumulation. (F) Differences in the in vivo tumor persistence of oxaliplatin after delivery by worm-like micelles vs. the free drug formulation in the SQ PDAC model.