Figure 6. UGDH knockdown inhibits GBM growth and migration in vivo.
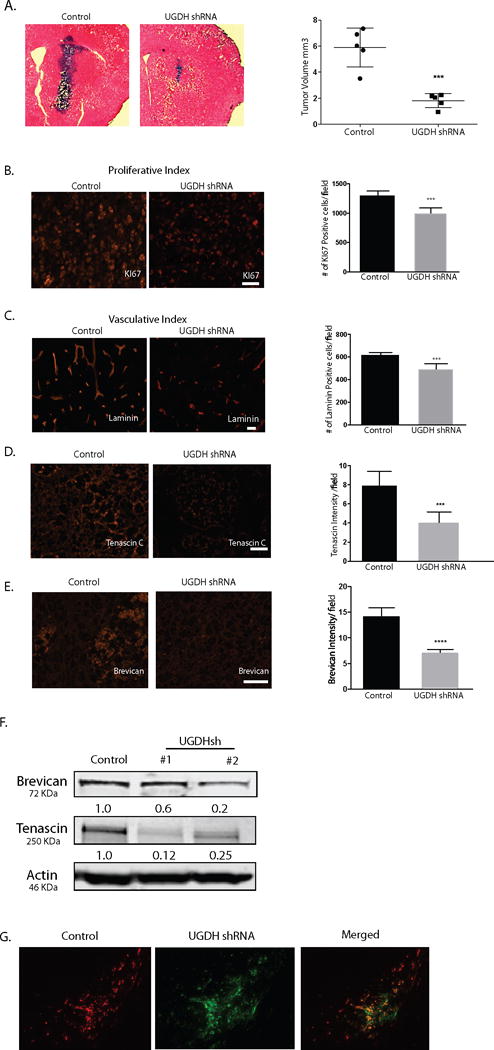
(A) Control shRNA or UGDH shRNA transduced GBM1A cells (100,000) were implanted by stereotactic injection to caudate/putamen of severe combined immunodeficiency mice (SCID). Animals were sacrificed 50 days after implantation. Hematoxylin and eosin-stained coronal brain sections (20 μm) obtained from animals showed dramatically decreased tumor size in UGDH knockdown groups (left panels, bar = 500 μm). Right panel: quantification of xenograft tumor volume shows that silencing UGDH repressed xenograft growth by more than 65% (6.4 in control vs. 2.3 in UGDH sh#1, P < 0.001). B. UGDH knockdown significantly inhibited tumor cell proliferation by 31% as evidenced by Ki67 staining. C. UGDH loss-of-function inhibited blood vessel density by 25% as evidenced by laminin staining. (D, E) Xenografts with UGDH knockdown decreased the abundance of key extracellular matrix components tenascin C and brevican in GBM1A xenografts. B to E: Bar = 20 μm. (F) Western blots showing decreased brevican and tenascin C protein in U87 UGDH knockdown cells in vitro. (G). A mixture of RFP-labeled control non-silencing cells (50,000) and GFP-labeled UGDH knockdown cells (150,000) was injected into the mouse brain. Dual-fluorescence experiment showed that UGDH knockdown cells (green) stayed in the tumor core (arrowheads), whereas the control cells (red) were preferentially located in the tumor periphery (arrows). Bar = 200 μm.
