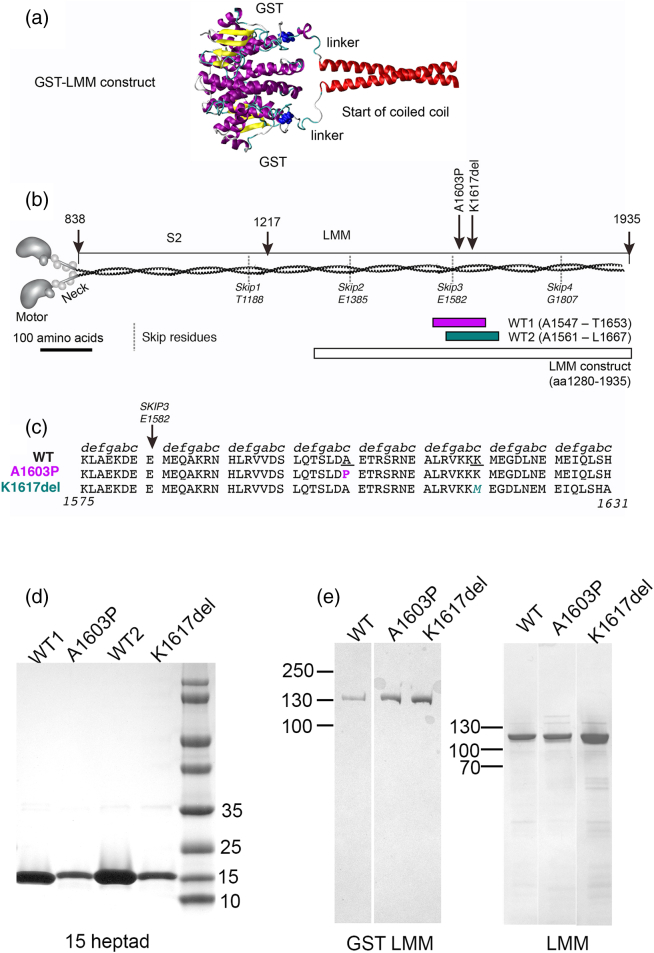
Fig. 1.
Mutant constructs. (a) A molecular model to show how GST is fused to the start of the coiled coil, in the GST–LMM constructs. Yellow: β-sheet, purple: α-helix, blue: C-terminus of GST and linker. (b) The positions of the two mutations studied here and the positions of the two 15-heptad constructs with respect to the full-length myosin, as well as the LMM construct used here, with amino acid numbers as indicated. Nominal positions of the four “skip” residues are shown. (c) Partial sequences of β-MHC, showing the WT sequence, and the mutant sequences for A1603P and K1617del underneath. The heptad assignments for WT are indicated as shown and positions of mutations as indicated. (d) SDS-PAGE gels of purified 15 heptad (15H) constructs. (e) SDS-PAGE gels of purified GST–LMM constructs and purified LMM constructs after GST has been proteolytically removed.