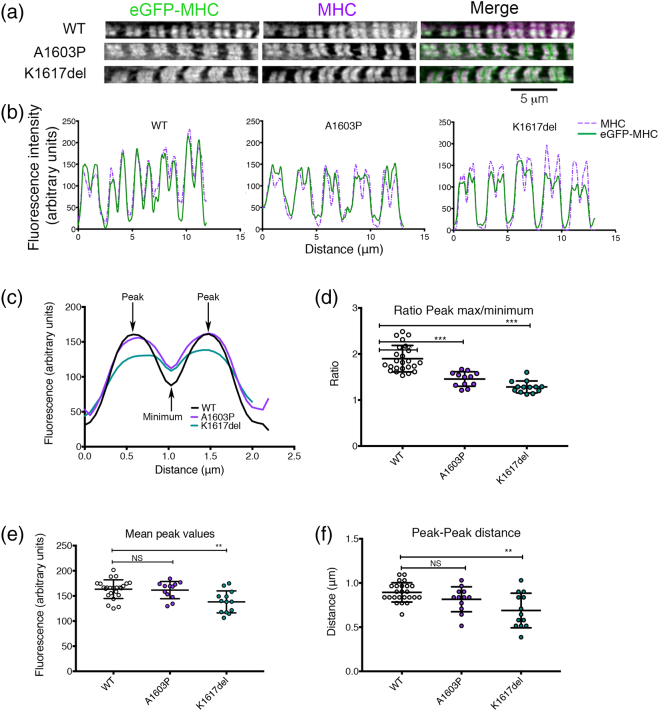
Fig. 6.
Mutant isoforms of eGFP–MHC affect incorporation into muscle sarcomeres in cultured skeletal muscle myotubes formed by C2C12 cells. (a) Images of individual myofibrils within cultured skeletal muscle myotubes expressing eGFP–MHC constructs as shown and co-stained for skeletal myosin using the A4.1025 antibody. (b) Example line profiles for MHC (magenta) and eGFP–MHC (green) across five sarcomeres from the images shown in panel a. (c) Mean fluorescence intensity profiles for eGFP–MHC organization across a single sarcomere, for WT and each mutant. The mean plot profile was calculated from measurements of at least 12 myofibrils (with 2–5 sarcomeres measured individually per myofibril) from different myotubes (n = 24, WT; n = 12, A1603P; n = 13, K1617del). Positions of the peak intensity values and the minimum intensity value (at the M-line) are indicated for WT eGFP–MHC. (d) The ratio between the mean peak intensity values (as in panel c) and the mean minimum intensity value at the M-line. (e) The mean peak values (averaged for both peaks, either side of the M-line) for WT eGFP–MHC and each of the mutants. (f) The mean peak–peak distance between peak maxima either side of the M-line. In panels d–f, each data point shows the average value for a single myofibril. The mean ± S.D. for each mutant is also shown overlaid as bars. Two-tailed t tests were used to compare mean values between the WT and mutant constructs (* p < 0.05, ** p < 0.001, *** p < 0.0001).