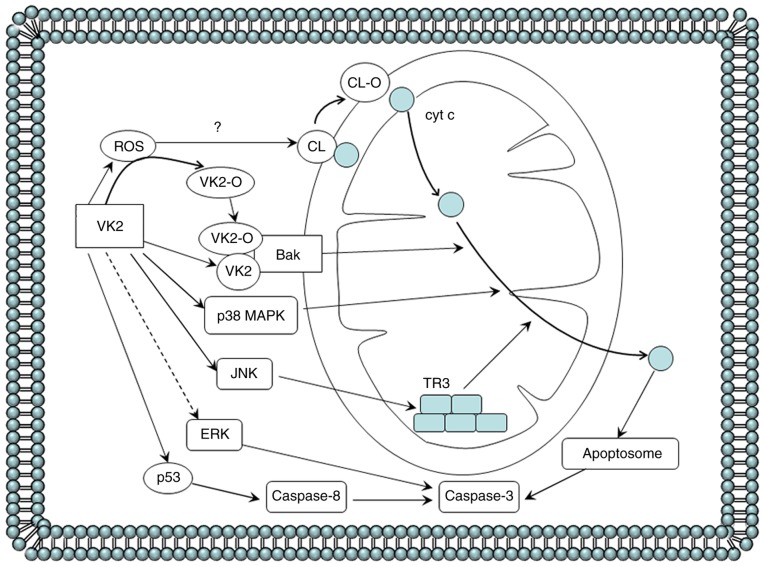
Figure 2.
Cell apoptosis induced by VK2 in cancer cells. VK2 induces apoptosis in cancer cells by depolarizing the mitochondrial membrane potential, followed by cytochrome c release from the mitochondria into the cytosol to form apoptosomes, which then activates caspase-3. The precise mechanism of VK2-dependent initiation of the mitochondrial apoptosis pathway of cancer cells is as follows. In HL-60 leukemia cells, VK2 and VK2-O selectively binds to the mitochondrial protein Bak. VK2-induced ROS generation prior to the induction of apoptosis possibly contributes by converting VK2 to VK2-O. In myeloma cells, VK2 activates p38 MAPK to its phosphorylated form. VK2 exposure in PA-1 ovarian cancer cells may activate JNK to phosphorylate TR3, also known as Nur77 and neuron growth factor inducible factor I-B, and increase TR3 levels in the mitochondria. The hypothesis that the release of cytochrome c from the mitochondria partly results from the acidic phospholipid CL being peroxidated by ROS is yet to be confirmed. The role of ERK in the VK2-dependent activation of caspase-3 and induction of apoptosis in hepatocellular carcinoma and pancreatic cancer cells is contradictory, so this pathway is represented with a dashed line. VK2 can inhibit ERK phosphorylation by suppressing the Ras activation and subsequently suppressing the catalysis of MEK, which causes apoptosis in HCC cells. Conversely, VK2-dependent induction of pancreatic cancer cell apoptosis is primarily associated with an increase in levels of phosphorylated ERK. In addition, VK2 stimulates the extrinsic apoptosis pathway by increasing p53 phosphorylation and then activating caspase-8 in Smmc-7721 HCC cells. Bak, Bcl-2 antagonist killer 1; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2 associated X protein; CL, cardiolipin; HCC, hepatocellular carcinoma; VK2, vitamin K2; VK2-O, VK2-2,3 epoxide; ROS, reactive oxygen species; MAPK, mitogen-activated protein kinase; JNK, c-Jun N-terminal kinase; MEK, MAPK kinase; ERK, extracellular-signal-related kinase.