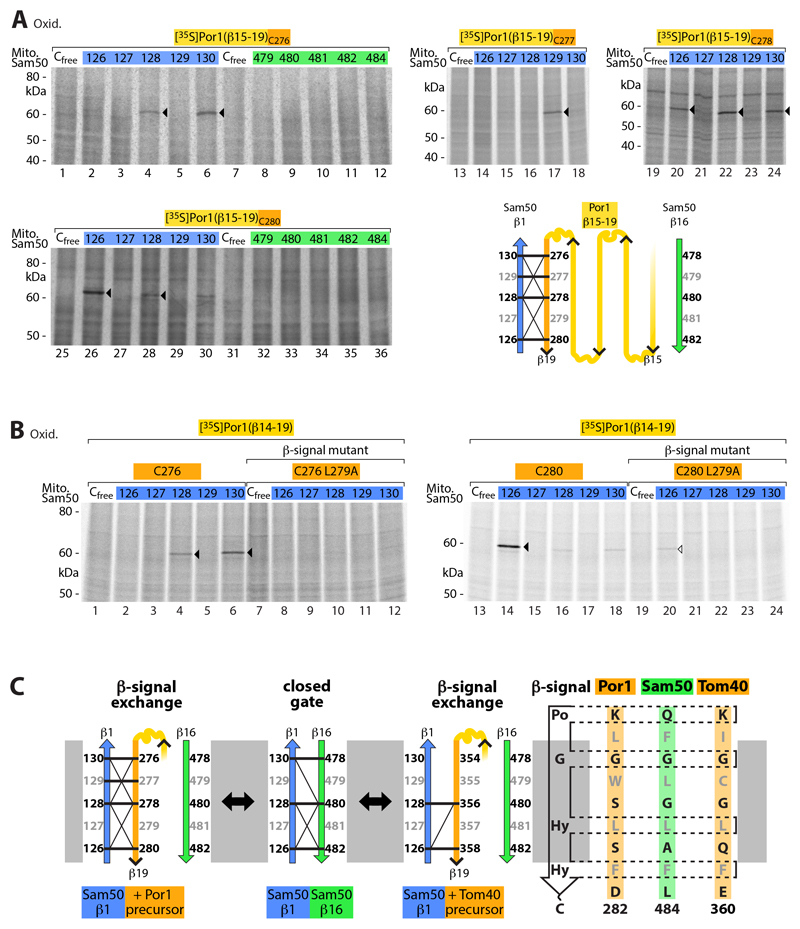
Fig. 2. Interaction of Sam50 β-strand 1 with the C-terminal β-signal of precursor proteins.
(A) Radiolabeled Por1(β15-19) precursors containing one cysteine at the positions indicated were imported for 5 min into mitochondria isolated from yeast strains expressing Sam50 with the indicated cysteine residues, followed by oxidation with 4-DPS (lanes 1-12, 19-36) or CuSO4 (lanes 13-18). Samples were analyzed by non-reducing SDS-PAGE and autoradiography. Arrowheads, disulfide-bonded Sam50-Por1(β15-19) adducts. Schematic model, disulfide bond formation of Sam50 β-strand 1 with the β-signal (β19) of the Porin precursor β15-19; thick and thin lines indicate strong and weak formation of Sam50-Por1 adducts, respectively. (B) [35S]Por1(β14-19)C276, [35S]Por1(β14-19)C280 and the corresponding β-signal mutants (L279A) were incubated for 5 min with isolated mitochondria of Sam50 cysteine variants followed by oxidation with 4-DPS, non-reducing SDS-PAGE and autoradiography. Arrowheads, cysteine-specific Sam50-precursor adducts. (C) Schematic model illustrating the β-signal exchange observed in Fig. 2 and fig. S2.