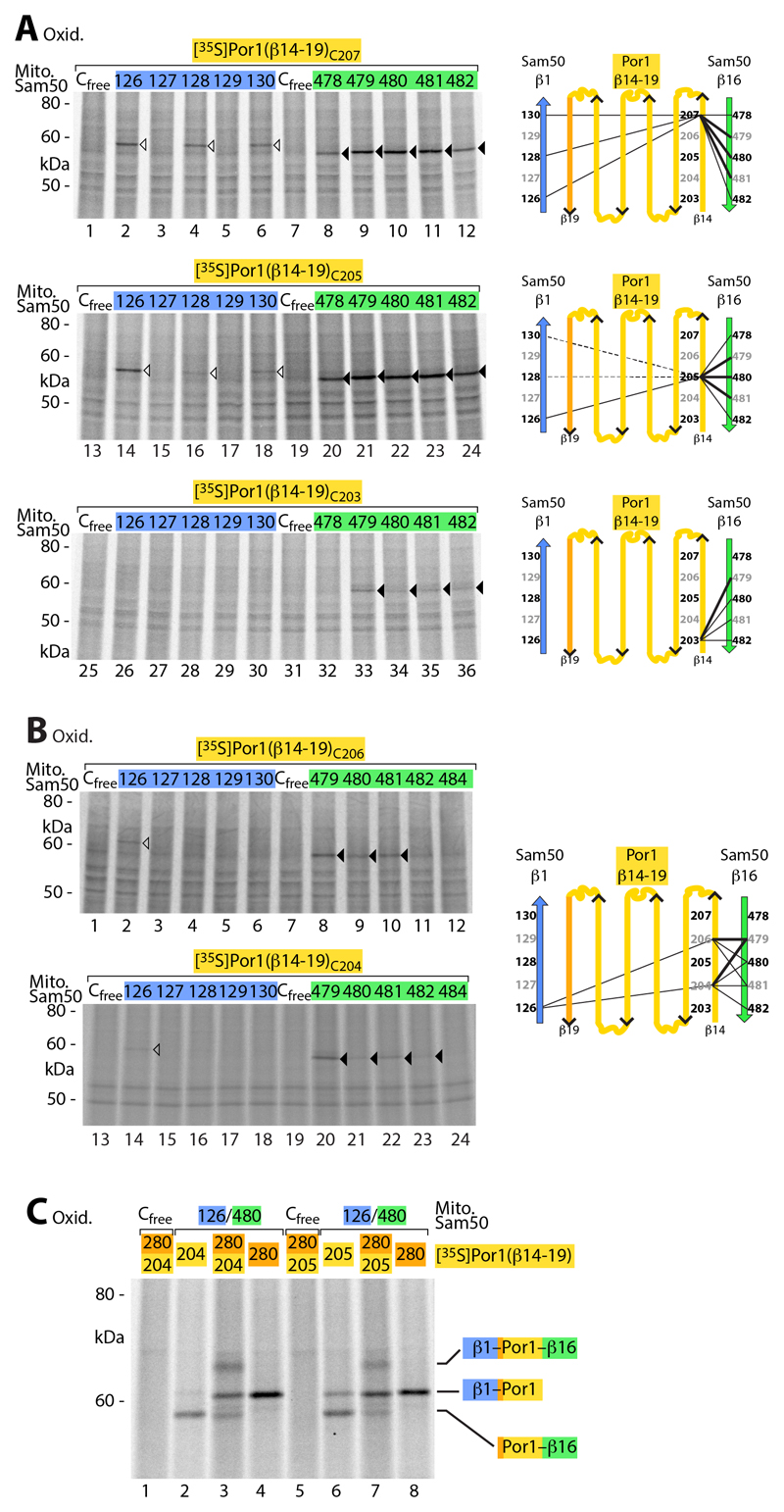
Fig. 4. Interaction of Sam50 with the N-terminal β-strand of precursor proteins.
(A) [35S]Por1(β14-19) precursors containing a single cysteine residue as indicated were imported into mitochondria isolated from yeast strains expressing the indicated Sam50 cysteine variants, followed by oxidation with 4-DPS, non-reducing SDS-PAGE and autoradiography. Black and white arrowheads, cysteine-specific disulfide-bonded Por1(β14-19) adducts to the C-terminal and N-terminal β-strand of Sam50, respectively. Right, schematic models. (B) [35S]Por1(β14-19)C206 and [35S]Por1(β14-19)C204 were treated as described in (A). (C) [35S]Por1(β14-19) single and double cysteine variants were incubated with isolated mitochondria from yeast strains expressing Sam50Cfree or the double cysteine variant Sam50C126/C480, followed by oxidation with 4-DPS. Samples were analyzed as described in (A).