Fig. 1. Cryo-EM reconstruction and atomic modeling of the HSV-1 capsid.
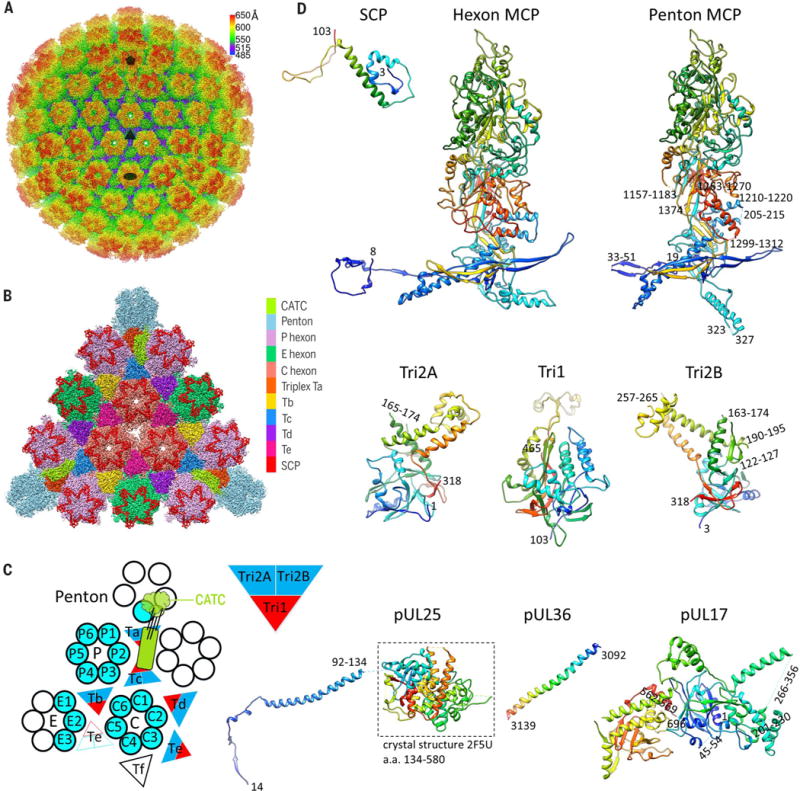
(A) Radially colored cryo-EM density map of the HSV-1 capsid viewed along a threefold axis. Fivefold, threefold, and twofold axes are denoted by a pentagon, triangle, and oval, respectively. (B) Magnified view of one facet of the icosahedral capsid with structural components differentially colored. The density of triplex Tf at the center is not shown. (C) A schematic representation of one asymmetric unit (shaded) of the capsid. An extra copy of triplex Te (unshaded) from an adjacent asymmetric unit is shown to depict that triplex pair Tb-Te has a similar configuration as Ta-Tc, thus providing a second potential binding site for the CATC (11, 17). The enlarged red and blue triangle shows the heterotrimeric nature of a triplex. a.a., amino acid. (D) Atomic models of individual capsid or tegument proteins in rainbow-colored ribbon (from blue at the N terminus to red at the C terminus). Numbers denote chain termini or flexible segments that are not modeled. a.a., amino acid.
