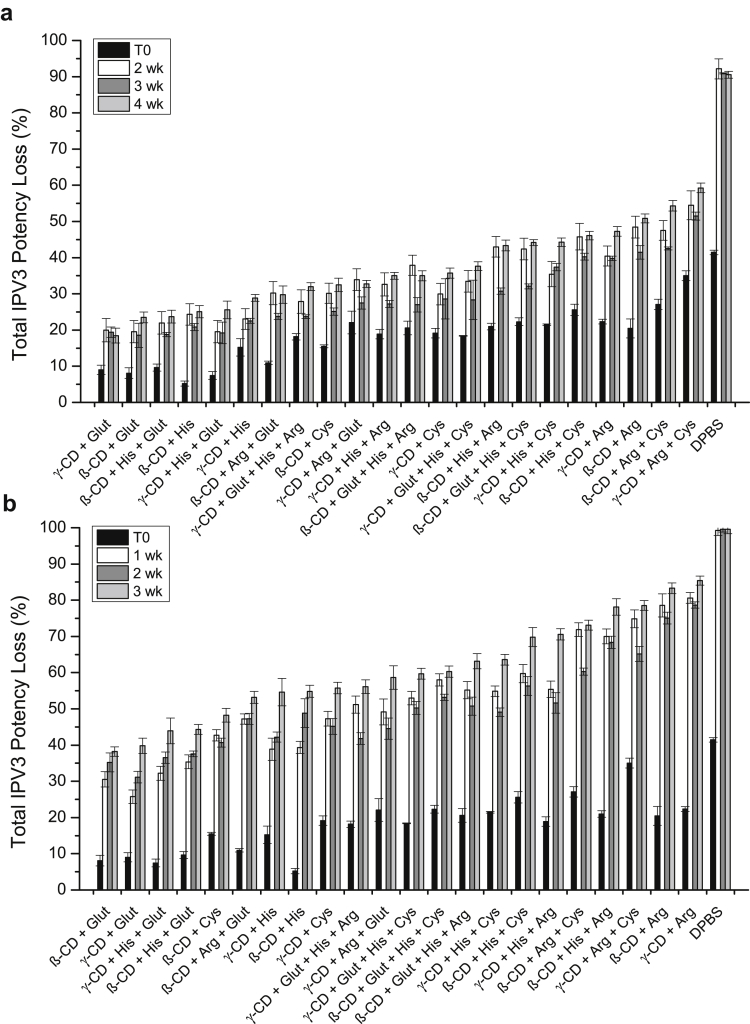
Figure 5.
Effects of optimized excipient combinations on the stability of IPV3 in tIPV samples during drying and storage in dried state on LCP discs. Each optimized tIPV formulation was stored for either 2, 3, or 4 weeks at 4°C (a), or 1, 2, or 3 weeks at 25°C (b) and the measured relative D-antigen potency loss was determined compared with a liquid tIPV stock solution (0% potency loss). T0 represents relative potency loss immediately after drying. Excipients tested in a M199/DPBS base buffer (see methods section): β-CD, 4.5% w/v SBE-β-cyclodextrin; Glut, 15-mM glutathione; His, 30-mM histidine; γ-CD, 2.5% w/v γ-cyclodextrin; Arg, 0.15-M arginine; Cys, 20-mM cysteine. Excipients were sorted from the lowest potency loss to highest loss at the last tested time point. Error bars represent the SD (1 SD) from triplicate experiments.