Figure 1. Characterization of the R201C mutant of Gαs.
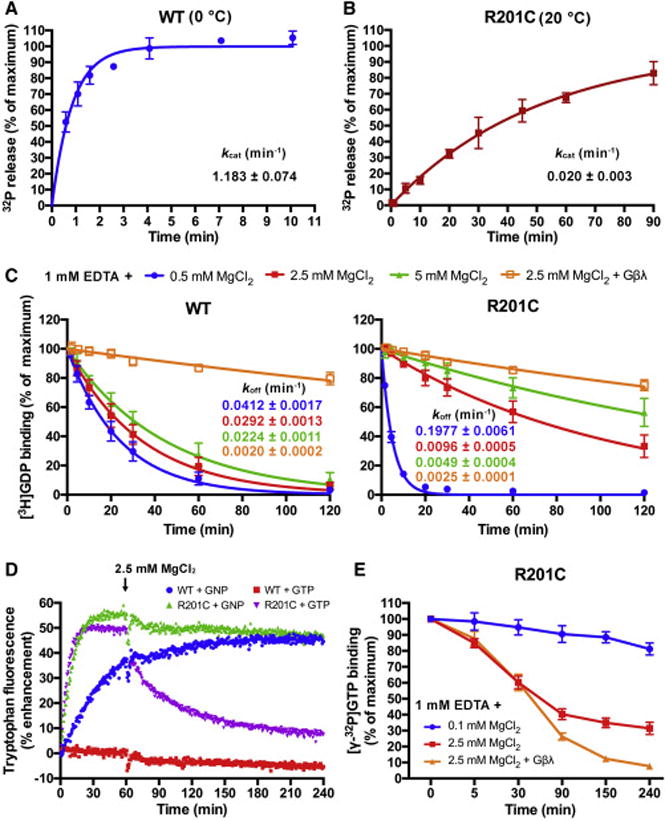
(A and B) The single turnover GTP hydrolysis rates (kcat) of wild type (WT) Gαs and the R201C mutant. Purified Gαs in a Mg2+-free or low Mg2+ buffer was first incubated with [γ-32P]GTP at the indicated temperature, then high concentration of Mg2+ and GTP were added to initiate the hydrolysis. 32PO43− release was quantified using liquid scintillation counting. The data represents the mean ± SD of four (WT) or three (R201C) independent measurements.
(C) Influence of MgCl2 and Gβγ subunits on the rates of GDP dissociation (koff) from WT Gαs and the R201C mutant. Gαs preloaded with [3H]GDP was assayed in a buffer containing 1 mM EDTA, 0.5 mM GDP and the indicated concentration of MgCl2 with or without 1 μM Gβ/γ(C68S). The data represents the mean ± SD of three independent measurements.
(D) The changes in the intrinsic tryptophan fluorescence of WT Gαs and the R201C mutant caused by binding of GNP, or binding and hydrolysis of GTP. 5 μM GDP-bound Gαs was mixed with 0.5 mM GNP or GTP in a buffer containing 1 mM EDTA and 0.1 mM MgCl2 to initiate the nucleotide exchange; after 1 hour, MgCl2 was added to a final concentration of 2.5 mM to decrease the GDP dissociation rates.
(E) Evaluation of the GTP occupancy of the R201C mutant in the presence of excess GTP. The R201C mutant was incubated with 20 nM [γ-32P]GTP and 400 μM GTP in a low Mg2+ buffer until the binding of [γ-32P]GTP to the R201C mutant reached a maximum, and the concentration of bound [γ-32P]GTP was measured and defined as the zero time point. Then MgCl2 or MgCl2 together with Gβ/γ(C68S) was added immediately, and the concentration of bound [γ-32P]GTP was measured at various time points. The data represents the mean ± SD of three independent measurements.
See also Figure S1.
