Figure 3. The phosphate-binding residues of arrestins.
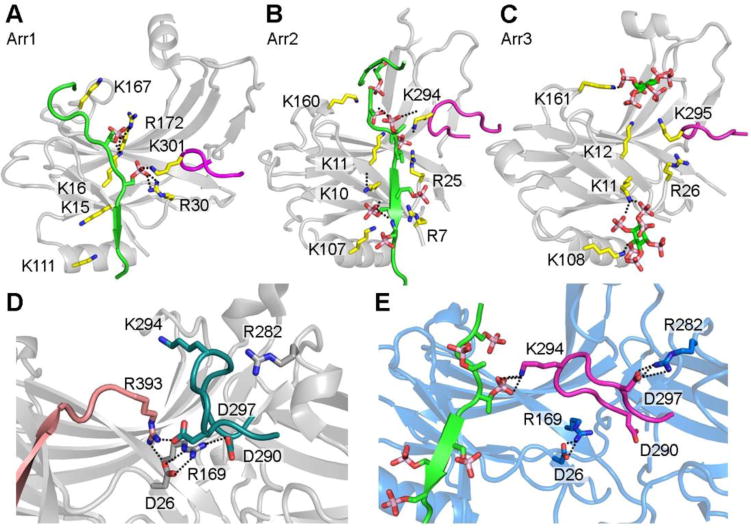
A. Phosphorylated receptor C-terminus binding sites within the arrestin-1 N-domain (PDB entry 5W0P). B. Phospho-peptide binding sites within the arrestin-2 N- domain (PDB entry 4JQI). C. IP6 binding sites within the arrestin-3 N-domain (PDB entry 5TV1). D. Conformation of the lariat loop in basal arrestin-2 (PDB entry 3P2D) with an intact polar core. The lariat loop contributes two charged residues to the polar core (D290 and D297) and also contains one phosphate-binding residue (K294). E. Conformation of the lariat loop in phospho-peptide activated arrestin-2 (PDB entry 4JQI) with the polar core disrupted. The lysine 294 binds to one phosphate from the receptor C-terminus phospho-peptide, which leads to the conformational change in lariat loop and the disruption of the polar core. The salt bridge is shown by dotted line.
