Figure 5. Switch regions of arrestin-3.
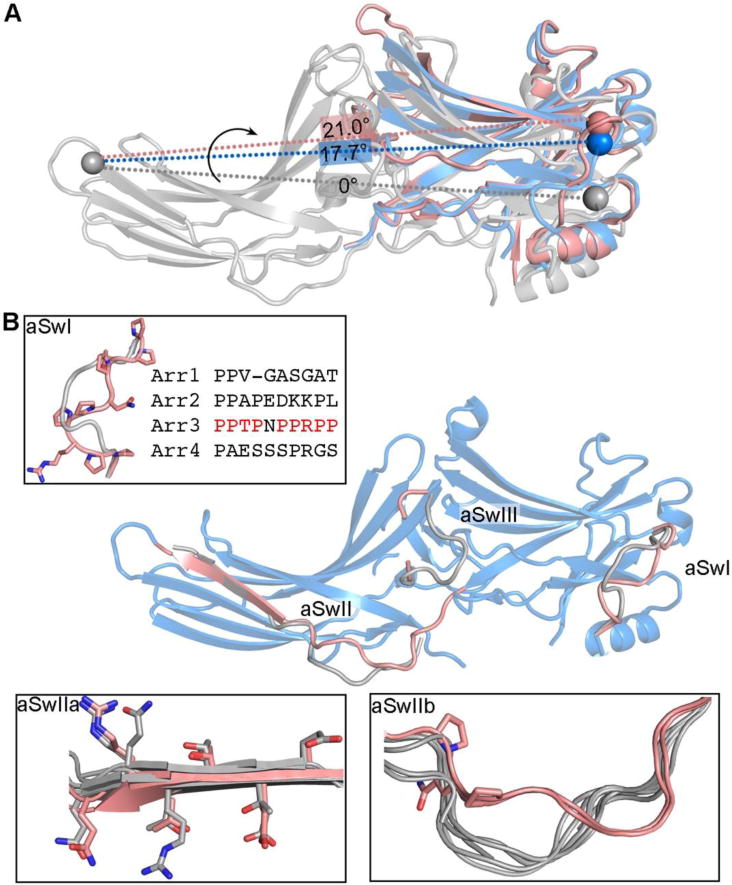
A. The inter-domain twist realigns effector-binding sites that span two domains. The spheres mark equivalent residues. The alignment of two potential effector bindings sites (spheres) is different in basal arrestin-3 (colored grey, PDB entry 3P2D), active arrestin-3 (colored blue, PDB entry 3P2D) and active arrestin-2 (colored pink, PDB entry 4JQI) due to different rotational angle between two domains. For an effector, which engages multiple binding sites spanning both domains, the change in the alignment of these binding sites can lead to the change of its affinity for arrestin. B. Conformations of the arrestin-3 switch regions in the active state (colored pink, PDB entry 5TV1) differs from the one in the basal state (colored grey, PDB entry 3P2D). The switch regions are enlarged in the insets.
