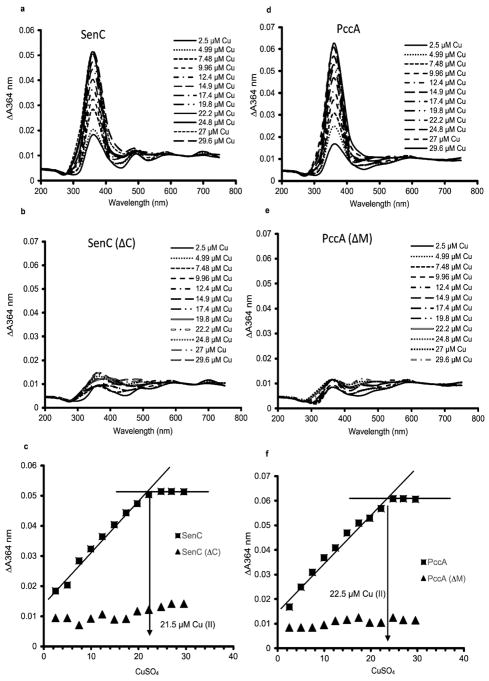
Figure 3.
Determination of the Cu binding stoichiometries of SenC and PccA. (a) Purified SenC (23 μM) was incubated with increasing concentrations of CuSO4 (2.5 to 30 μM), and the absorbance spectra were recorded between 200 and 750 nm. Cu-free SenC (as purified) was used to record the baseline. (b) The same experiment as in (a) was repeated with 23 μM SenC(ΔC), a purified mutant variant of SenC lacking the conserved Cys residues of its Cu binding motif. (c) The absorbances at 364 nm (indicative of a Cu(II)–thiolate charge transfer complex) measured in (a) and (b) were plotted against the CuSO4 concentrations used for determining the Cu(II) binding stoichiometry of SenC. Binding saturation was reached at ~21.5 μM Cu(II), indicating a 1:1 Cu(II):SenC stoichiometry. No significant Cu binding was detected with SenC(ΔC). (d) As in (a) except that purified PccA (23 μM) was used. (e) As in (d) except that 23 μM PccA(ΔM), a purified PccA variant lacking the two conserved Met residues of its Cu binding motif, was used. (f) As in (c). Cu(II) binding saturation was reached at ~22.5 μM Cu(II), indicating a 1:1 Cu(II):PccA stoichiometry. No significant Cu binding was detected with PccA(ΔM).