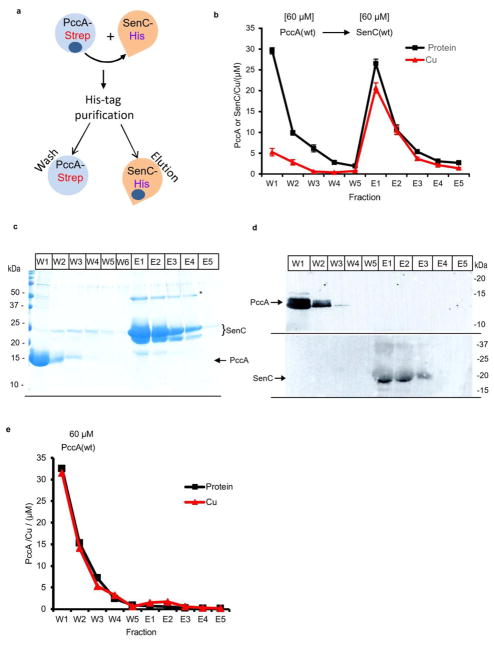
Figure 5.
PccA transfers Cu(II) to SenC. (a) Schematic view of the Cu transfer assay used in these experiments. In brief, Strep-tagged PccA was loaded with Cu and incubated with Cu-free His-tagged SenC. The mixture was purified via Talon, resulting in wash fractions containing PccA and elution fractions containing SenC. The protein and Cu contents of each fraction were then determined. (b) Cu(II)-loaded PccAStrep (60 μM) was incubated with Cu-free SenCHis (60 μM) for 10 min at room temperature, and the mixture was loaded onto a Talon affinity column. PccA was recovered in the flow-through and wash fractions (W1–W5) and SenC in the elution fractions (E1–E5) (see the Supporting Information). For each fraction, the protein content was determined by the Lowry method and converted to molarity using the molecular weight of PccA for fractions W1–W5 and that of SenC for E1–E5. The Cu content of each fraction was determined by the BCS method after SDS denaturation and reduction to Cu(I) by ascorbate, as described in the Supporting Information. (c) Coomassie staining of the wash and elution fractions and (d) immune detection using antibodies against PccA or SenC. Cu transfer experiments were repeated three times. (e) A control experiment as in (b) but in the absence of the acceptor protein SenC indicated that a negligible amount of Cu was retained by the column matrix used.